Major depressive disorder (MDD) remains a pervasive global health challenge, affecting millions worldwide and ranking among the leading causes of disability. MDD leads to substantial healthcare costs and contributes heavily to the overall disease burden (1). Despite the widespread use of antidepressant medications, many patients do not achieve sustained relief. As an alternative, neuromodulation therapies such as electroconvulsive therapy (ECT) play a vital role. ECT is a well-established intervention that demonstrates exceptional efficacy in multiple psychiatric disorders, including MDD. It involves the administration of electrical currents, either unilateral or bilateral electrode placements on the patient’s head, to induce a brief, controlled seizure. Ultimately, this process is thought to elicit reorganization of key cortical networks involved with mood and cognition. However, ECT can cause significant adverse effects, such as memory loss and confusion, rendering ECT to be reserved for severely treatment resistant patients (2, 3). Identifying the specific neurophysiological changes induced by ECT could lead to the development of safer and more effective treatments. While the optimal stimulation methods and parameters are still being investigated, ECT remains essential for managing treatment-resistant depression.
More than eight decades have passed since its introduction as a clinical intervention, yet the precise neurobiological mechanisms underpinning ECT’s therapeutic effect remain elusive. Current research suggests that ECT’s benefits are likely achieved through multiple mechanisms. These include, but are not limited to, changes in neurotransmitter transmission, enhancement of neurotrophic and neuroplastic activities, modulation of cortical networks, reduction of neuroinflammation, and regulation of the endocrine system (3–10). Given the historical precedence of the monoaminergic theory of depression, a plethora of studies in both animals and humans have sought out to determine whether ECT’s efficacy is related to changes in serotonergic activity (11, 12). While the evidence remains inconclusive, ECT appears to have some notable effect on serotonergic neurotransmission (3). A significant challenge in this area is that peripheral biomarker measurements do not reliably reflect neurotransmitter levels in the brain. Advanced neuroimaging techniques can be employed to gather insight into the effect of treatments such as ECT on neurotransmitter activity.
Loudness dependence of auditory evoked potentials (LDAEP) is a method used to measure the response of cortical potentials to variations in the intensity (i.e., loudness) of auditory stimuli. LDAEP is typically assessed using electroencephalography (EEG). The relationship between stimuli loudness and evoked potential amplitudes in the primary auditory cortex has been suggested as an indicator of serotonergic neurotransmission (13). Serotonin is thought to play a role in auditory processing, as layer IV of the primary auditory cortex is densely innervated with serotonergic fibers originating from the raphe nucleus (14, 15). Initial studies in animal models reported that higher serotonin activity was correlated with less dependence on stimulus intensity (i.e., similar amplitudes in cortical evoked potentials regardless of loudness). Conversely, lower serotonin activity was correlated with loudness-dependent changes in evoked potential amplitudes (13, 16). Furthermore, LDAEP has been proposed to be a protective mechanism in auditory processing, which helps prevent overstimulation and excitotoxicity (17). Within the primary auditory cortex, the neurobiological mechanisms of a reduced LDAEP being associated with high serotonin activity is proposed to rely on serotonergic modulation of cortical excitability. This modulation occurs indirectly via GABA-ergic interneurons, which express excitatory 5-HT2A receptors, and directly via pyramidal cells, which express both excitatory 5-HT2A and inhibitory 5-HT1A receptors (13, 16, 18, 19).
An abundance of literature now exists exploring the relationship between the LDAEP and other neurotransmitter systems and biomarkers (20, 21). While evidence for the LDAEP’s relationship with serotonin is controversial, it is clear serotonergic activity serves a critical role in the functioning of the primary auditory cortex (20, 22–26). Numerous studies provide robust support for the influence of serotonergic activity on neuronal functioning across auditory processing pathways (27–30). Notably, a recent positron emission tomography (PET) study on the molecular mechanisms underlying the LDAEP reported that this biomarker is strongly and positively correlated with 5-HT1A binding in the temporal cortex, specifically in the location of the primary auditory cortex (15).
Additionally, studies have demonstrated that serotonin plays a crucial role at the intersection of psychiatric disorders and auditory conditions, including tinnitus and hearing loss (27, 28). In particular, MDD has been reported to be associated with impaired auditory processing. Studies indicate that deviations in serotonergic activity are evident in the auditory cortices of individuals with depression compared to controls (25, 31–34). For example, increased 5-HT1A binding and decreased 5-HT2A binding specifically within the primary auditory cortex in depressed patients has been reported (25). Moreover, treatments for depression, including ECT, have been shown to have a significant effect on auditory processing, demonstrated via increased activity, excitability, and intrinsic connectivity within the auditory cortices (31, 32, 35–37). Additionally, ECT has shown a pronounced impact on auditory evoked potentials, further underscoring the complex interplay between serotonergic modulation and auditory functions in psychiatric contexts (38, 39).
Given the efficacy of serotonergic agents such as selective serotonin reuptake inhibitors, and more recently psychedelics like psilocybin, in the treatment of depression, it is likely regulation of this monoamine system serves a pivotal role in ECT’s efficacy (40). Studies show mixed results regarding ECT’s impact on serotonergic receptors. For instance, some reports indicate that electroconvulsive stimuli result in decreased binding and activity of both 5-HT1A and 5-HT2A receptors (12, 41–43). However, other studies reveal no change in 5-HT1A activity, an increase in 5-HT2 activity, and enhanced serotonin transporter (SERT) receptor levels following ECT (44–47). Despite these discrepancies, there is broad consensus that ECT has a robust impact on serotonergic receptors in the treatment of multiple psychiatric disorders (8, 12, 48, 49).
Conventional LDAEP studies typically employ EEG. A 1982 study by Hari et al. compared simultaneous magnetic (AEF) and electrical (AEP) responses to auditory tones, demonstrating that AEF measured by MEG are highly comparable to AEP measured by EEG for short interstimulus intervals (< 4s) (50). MEG can offer better spatial resolution than EEG, particularly for superficial cortical sources (50). This comparison provides a foundational basis for the application of MEG in auditory evoked potential studies. Furthermore, subsequent studies have confirmed the reliability and validity of using MEG to measure LDAEP (51).
This study utilizes high-resolution magnetoencephalography (MEG) to measure cortical activity and determine the LDAEP in individuals before and after ECT. The primary aim is to explore the changes in the central serotonergic neurotransmission attributable to ECT, by analyzing variations in LDAEP. We hypothesize that ECT will decrease LDAEP, indicative of enhanced serotonergic neurotransmission. This approach not only promises to deepen our understanding of the neurochemical environment in patients undergoing ECT but also sheds light on the neurobiological mechanisms that underpin ECT’s effectiveness.
2 MethodsStudy participants and ECT treatment Ethical approval was obtained from the Human Research Protections Office at the University of New Mexico (UNM) before study initiation. The research was conducted in full compliance with the ethical standards outlined in the Declaration of Helsinki. Patients were recruited from the UNM Mental Health Center’s inpatient and outpatient services. All patients either had the decisional capacity to consent or, where necessary, provided assent with a surrogate decision-maker giving formal consent. All patients completed a full course of electroconvulsive therapy (ECT) using the ultra-brief pulse width, right unilateral electrode placement as previously described (52). During the initial session, the seizure threshold was determined using a dose titration method, which then guided the dosage for subsequent treatments. Specifically, the stimulus dosage was set at six times the threshold. Treatments were administered thrice weekly, and continued until an adequate clinical response was achieved or a decision was made to cease treatment due to non-response.
2.1 MRIAll MRI scans were conducted using the 3-Tesla Siemens Trio scanner at the Mind Research Network (MRN). High-resolution T1-weighted structural images were acquired using a 5-echo MPRAGE sequence with the following parameters: echo times (TE) of 1.64, 3.5, 5.36, 7.22, and 9.08ms; repetition time (TR) of 2.53s; inversion time (TI) of 1.2s; a flip angle of 7°; a single excitation; a slice thickness of 1mm; a field of view of 256mm; and a resolution of 256×256. Structural MRI preprocessing and the delineation of structural images were conducted using FreeSurfer 4.5.0 software (https://surfer.nmr.mgh.harvard.edu) (53).
2.2 MEG acquisition and data processingPrior to and following the ECT course, patients underwent MEG scans (see Figure 1). MEG recordings were captured using the Elektra Neuromag VectorView 306 system, which is equipped with 102 magnetometers and 204 planar gradiometers. To ensure accurate alignment, the MRIs were coregistered with scalp fiducial markers. While seated inside the MEG helmet, patients were exposed to a series of auditory tones at five different intensity levels: 55, 65, 75, 85, and 95dB. The tones were emitted through biauricular earbuds at a frequency of 2kHz, lasting 50ms each. The tones were presented in a random sequence, with interstimulus intervals of 1.2–2s; each intensity block comprising 22 tones, resulting in a total of 110 trials per intensity level.
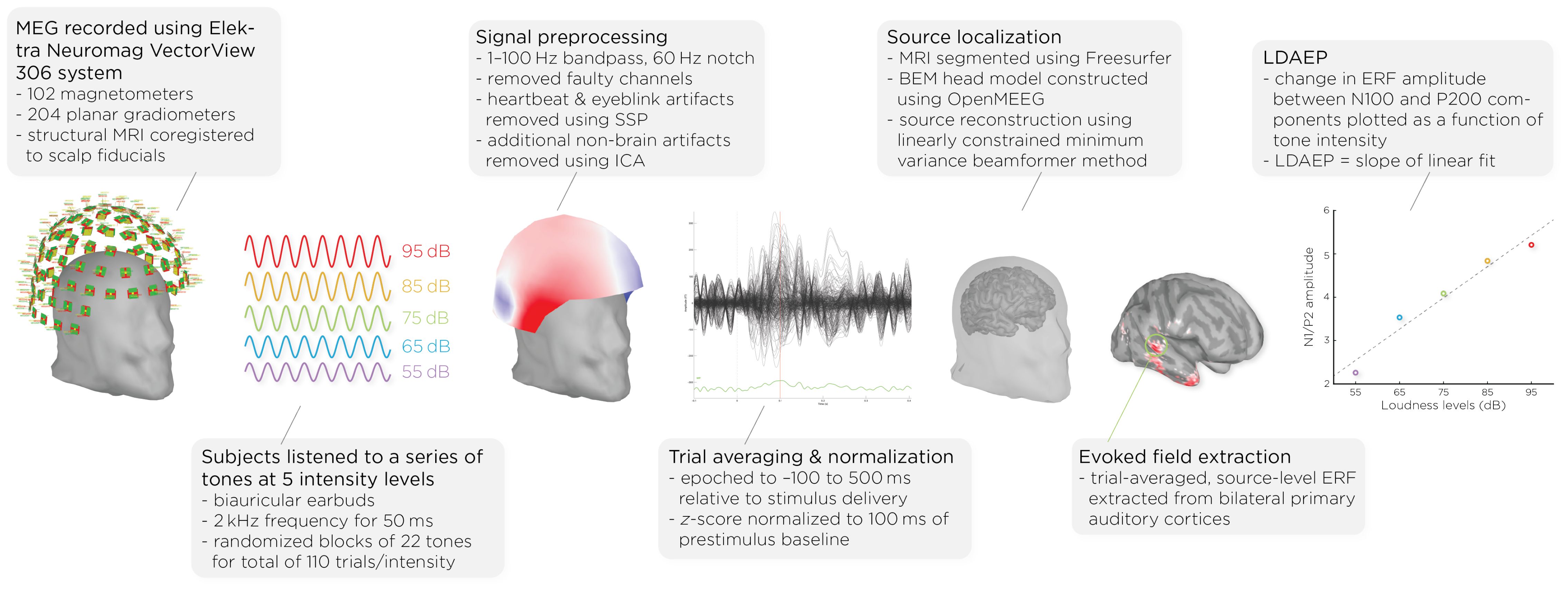
Figure 1. Workflow of MEG acquisition and data processing, detailing the steps from signal acquisition to data analysis. It shows the MEG setup, signal preprocessing, auditory stimulus presentation, data normalization, brain activity source localization, and the quantification of auditory evoked fields (AEFs) across different sound intensity levels.
Data analysis was performed with Brainstorm 3 (54), which is documented and freely available for download online under the GNU general public license (http://neuroimage.usc.edu/brainstorm). The MEG data was filtered using a 1–100Hz bandpass filter and a 60Hz notch filter to eliminate electrical line noise. Malfunctioning channels were identified and excluded. Artifacts arising from cardiac activity and eye blinks were removed via signal-space projection, and independent component analysis was used to eliminate other non-brain artifacts. Auditory events were defined for a time window from −100 to 500ms around the tone’s presentation. The data was normalized using the Z-transformation relative to the 100ms pre-stimulus baseline.
Cortical structures were derived from each subject’s MRI scans using FreeSurfer, and aligned with a standard brain atlas for cortical reconstruction. The head model for the forward model utilized the symmetric boundary element method (BEM) implemented in OpenMEEG, provided by the INRIA institute. This model established a computational link between the neuronal activity in the source space and the recorded MEG data in the sensor space, considering the conductive properties of head tissues. The inverse model, which infers neural activity from the MEG data, was computed using a data covariance matrix through the linearly constrained minimum variance (LCMV) beamforming technique, focusing on auditory evoked fields. Trial-averaged, source-level event-related fields (ERFs) were extracted from the bilateral primary auditory cortices. Finally, the LDAEP was calculated by evaluating the change in normalized ERF amplitude between the N100 and P200 components from the trial-averaged epochs. The LDAEP is represented by the slope of the linear regression line fitted to these data points.
2.3 Statistical analysisWe evaluated the distribution of our data for normality using the Shapiro–Wilk test. The tests indicated normality in the changes in the LDAEP slope (W = 0.86, p = .10), HDRS24 (W = 0.90, p = .26), and total RBANS scores (W = 0.84, p = .11). To investigate changes in LDAEP slopes, depression scores, and cognitive functioning scores before and after treatment, we employed paired t-tests. Additionally, we explored correlations among the pre-treatment and post-treatment LDAEP slopes, the degree of their changes, and the baseline, post-treatment, and changes in HDRS24 and RBANS scores. Initially, we attempted to include age and sex as covariates in a multiple linear regression model. However, given the small sample size of nine participants, which limited the statistical power and reliability of the estimates, and the lack of significant findings for age and sex, we decided to revert to simpler Pearson’s correlations.
3 Results3.1 Demographics and clinical outcomesThe study involved nine participants, six of whom were female, with an age range from 50 to 78 years. The average age of the participants was 68.1 years with a standard deviation of 10.7 years, and six participants were 65 or older. Prior to receiving treatment, the average score on the 24-item Hamilton Depression Rating Scale (HDRS24) for these patients was 37.2 (standard deviation = 12.8). Additionally, the mean score on the total Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was 82.9 (19.4). Six of nine patients responded (> 50% reduction in HDRS24 from baseline) to ECT with an average post-ECT HDRS24 score of 9.1 ± 7.6 (t8 = 5.60, p < .001). We collected seven of the nine patients’ RBANS data, and found, on average, their cognitive functioning did not change with ECT (t6 = 0.36, p = .73). Demographics and clinical measures before and after ECT treatment are summarized in Table 1.
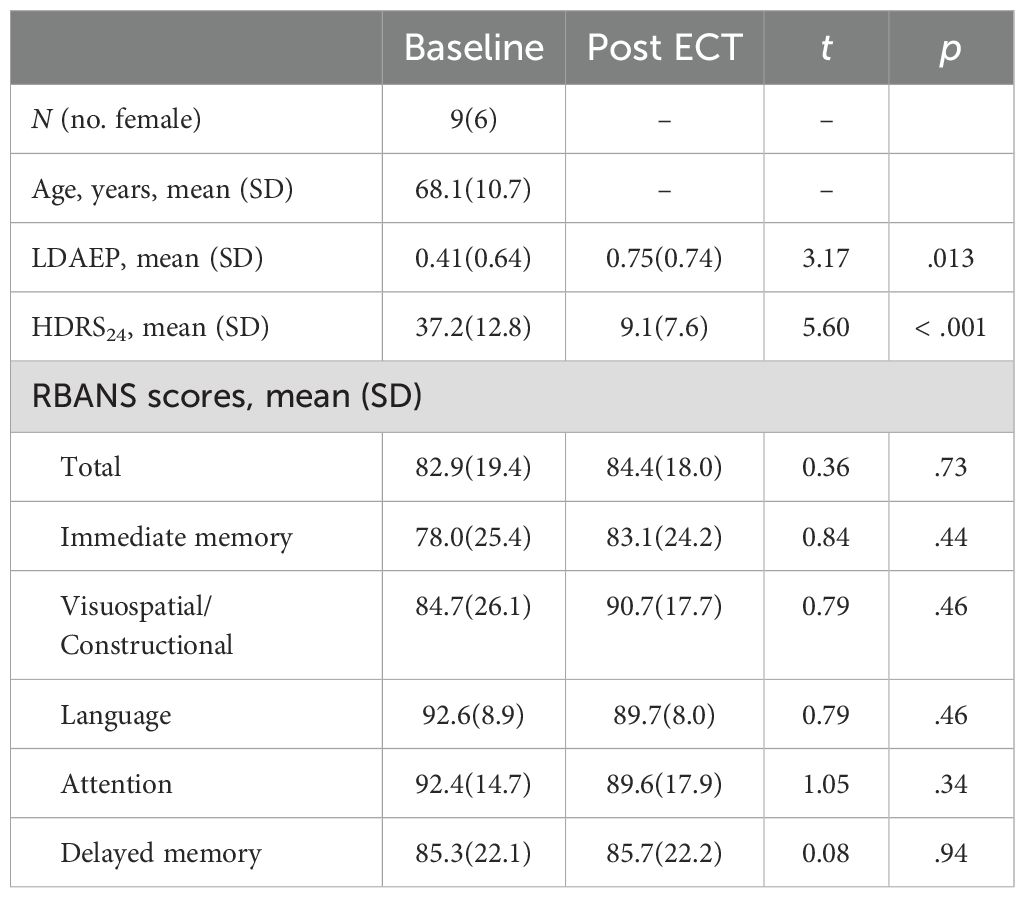
Table 1. Demographics and clinical measures before and after ECT treatment.
3.2 Change in LDAEPFigure 2 shows the auditory evoked fields before and after ECT treatment, with responses at varying stimulus loudness levels. The LDAEP slope significantly increased following ECT treatment from 0.41 ± 0.64 to 0.75 ± 0.74) (Cohen’s d = 0.49, t8 = 3.17, p = .013) (Figure 3).
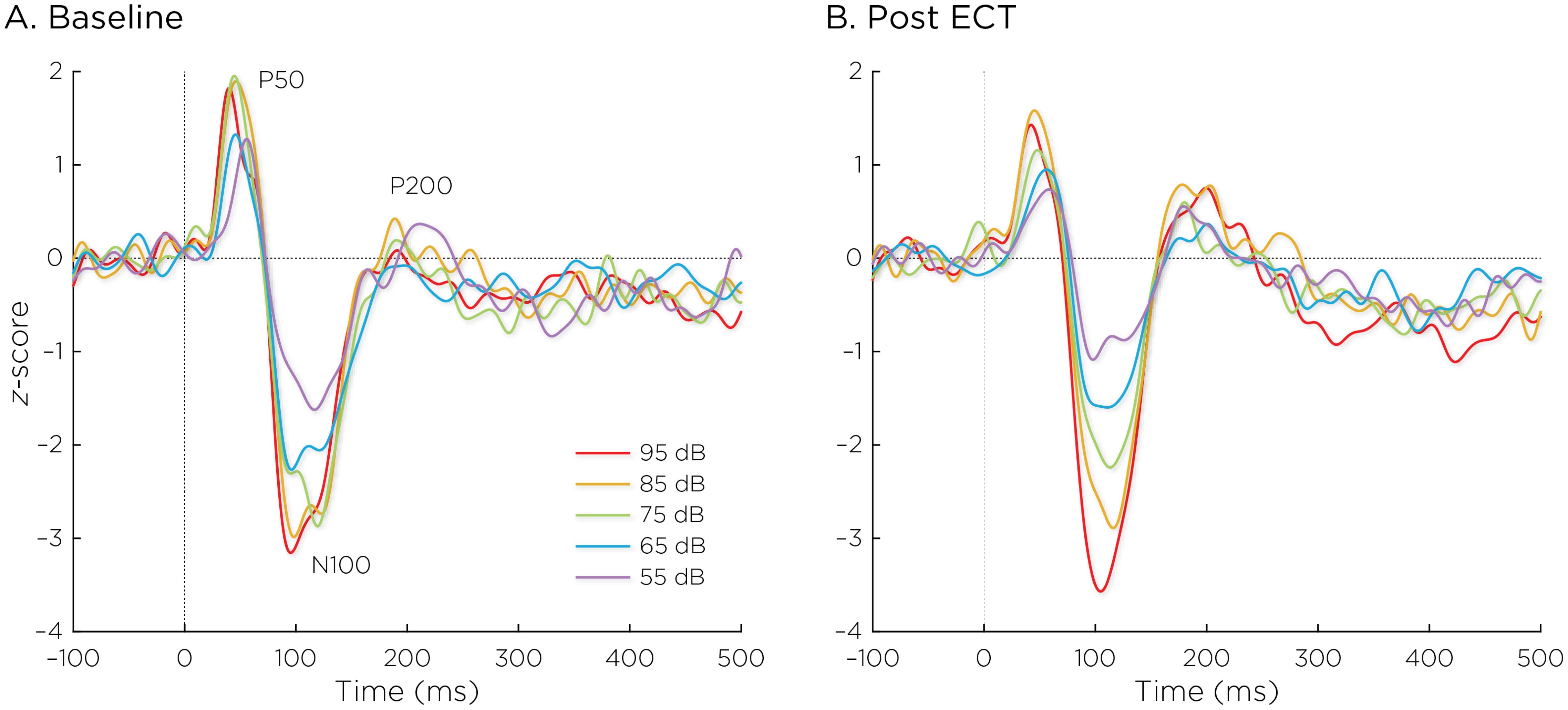
Figure 2. Auditory evoked fields (A) before and (B) after ECT treatment, with responses at sound pressure levels of 55–95dB. The change in normalized evoked field amplitude between the N100 and P200 (N1/P2) components of the trial-averaged epochs was calculated. The LDAEP is calculated as the slope of linear regression line that best fits the N1/P2 amplitudes at each sound pressure level.
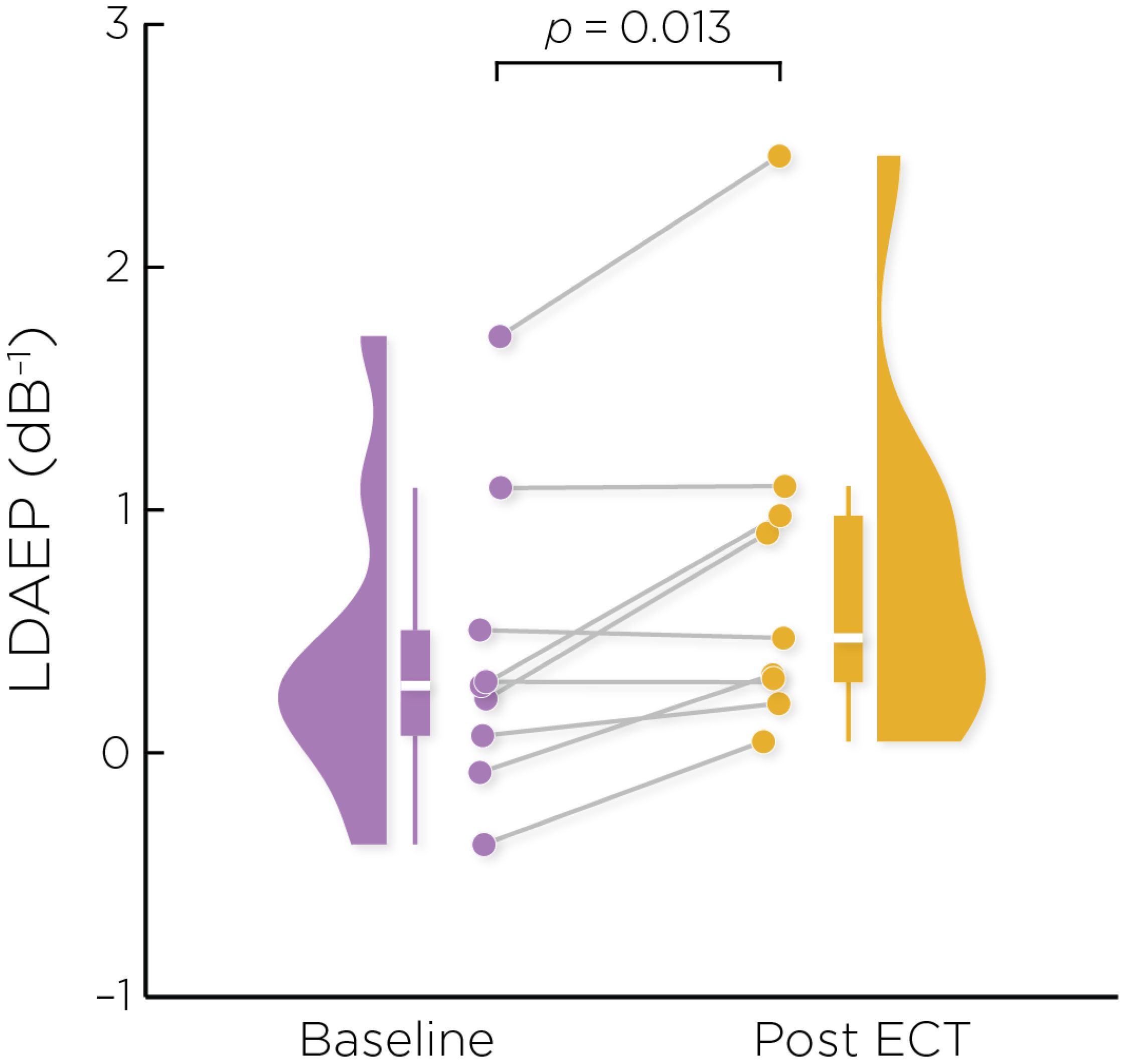
Figure 3. Individual changes in the pre- and post-ECT LDAEP slope measure. There was a significant increase post-ECT compared to baseline (Cohen’s d = 0.49, t8 = 3.17, p = .013).
3.3 Correlations between LDAEP and depression and cognition scoresThe change in the LDAEP slope was not significantly correlated with baseline (r = −0.079, p = .84), post-treatment (r = 0.066, p = .87), or changes in HDRS24 (r = 0.101, p = .80). The pre-ECT LDAEP slope was not significantly correlated with baseline (r = −0.194, p = .62) nor changes in HDRS24 (r = 0.339, p = .37).
We focused on assessing correlations with RBANS total scores. The change in LDAEP slope was not significantly correlated with the baseline (r = 0.208, p = .66), post-treatment (r = 0.403, p = .37), or changes (r = 0.276, p = .55) in the RBANS total score. The pre-ECT LDAEP slope was significantly correlated with the baseline RBANS total score (r = 0.855, p = .014), but not correlated with changes in the RBANS total score (r = −0.352, p = .44).
4 DiscussionIn this study, we used LDAEP as a cortical activity biomarker to monitor changes in neurotransmitter activity induced by ECT. Our initial hypothesis posited that ECT would mitigate symptoms of depression by boosting serotonergic neurotransmission, which would manifest as a reduced LDAEP, reflected by weakening of the response amplitude as a function of sound intensity levels. However, our findings revealed a significant increase in LDAEP post-ECT. Interestingly, the alterations in LDAEP did not correlate with changes in depression severity or cognitive performance.
The neurochemical underpinnings of LDAEP suggest that ECT should lead to a reduction in serotonergic tone within the primary auditory cortex, but this assumption is subject to debate. Studies on the relationship between LDAEP and serotonergic activity have harbored conflicting evidence and perspectives (21, 26, 55, 56). While numerous studies have found that the LDAEP is sensitive to acute changes in serotonergic activity, such as following administration of serotonergic-reuptake inhibitors, other studies have presented contrasting findings (57–63). A recent narrative review by Kangas et al. stated that LDAEP studies have generally yielded no consistent difference between depressed and non-depressed controls, though there appears to be a relationship with depression-subtypes (20). Given this context, a possible explanation for our results not supporting the original hypothesis could be that the LDAEP does not precisely mirror serotonergic tone in the primary auditory cortex.
Our findings did reveal a significant modulation of the LDAEP after ECT treatment. Intriguingly, the observed increase in LDAEP may indicate that ECT prompted a reduction in serotonergic receptor activity within the primary auditory cortex, particularly 5-HT1A and 5-HT2 receptors. This aligns with multiple studies demonstrating a reduction in 5-HT2 and 5-HT1A receptors in humans and non-human primates following ECT (41–43, 64, 65). The initial LDAEP studies in animal models bolster the plausibility of ECT-induced reduction in serotonergic receptors, in that a 5-HT1A agonist decreased the LDAEP and a 5-HT2A antagonist increased the LDAEP, suggesting that decreased serotonin receptor activity results in a strengthened LDAEP (13, 66). However, pre-clinical studies have largely found an upregulation of 5-HT1A and 5-HT2A receptors after electroconvulsive stimuli in animal models (45, 47, 67). This discrepancy underscores the need for further research to understand ECT’s impact on serotonergic receptor activity in humans more comprehensively.
Several alternative explanations as to why the LDAEP increased following ECT may be plausible. The LDAEP has been shown to co-vary with symptom severity in disorders such as ADHD, schizophrenia, and Parkinson’s disease, all of which are strongly linked to dopaminergic dysregulation (68–70). Given that ECT has been found to significantly alter dopaminergic neurotransmission (45, 71–74), one possible explanation for the heightened LDAEP could be ECT’s direct influence on dopamine receptor and transporter activities. Furthermore, considering that the LDAEP results from both excitatory and inhibitory post-synaptic potentials within the primary auditory cortex, the altered LDAEP could reflect changes in glutamatergic and GABAergic functions. Indeed, ECT has been shown to increase GABA concentration, normalize glutamate deficits, and alter excitation/inhibition ratios (28, 75–78). For instance, the administration of a glutamatergic NMDA antagonist has been reported to blunt LDAEP, suggesting that increased glutamatergic activity correlates with a heightened LDAEP (79). On the other hand, a study reported that the LDAEP is not associated with GABA levels (80). Further exploration of the relationships between ECT, auditory cortical activity, and excitatory and inhibitory neurotransmitters could yield valuable insights.
Beyond neurotransmitter effects, there is a consistent body of evidence indicating that ECT is associated with increased gray matter volume in the temporal lobes, including the superior temporal gyrus (5, 81–83), where the primary auditory cortex is situated. It is possible that neurotrophic effects are related to the increased LDAEP following ECT in this study. A recent meta-analysis concluded that ECT directly increases concentrations of brain-derived neurotrophic factor (BDNF) (84). Similarly, the LDAEP has been found to be significantly positively correlated with serum BDNF levels (85). One possibility is that ECT’s robust neuroplastic effects within the temporal lobe are related to the modulation of the LDAEP. Moreover, systematic reviews found that ECT has consistently been reported to decrease levels of inflammatory biomarkers, tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) (86, 87). Notably, one study demonstrated that the LDAEP was negatively correlated with TNF-α (88). This could suggest that a reduction in TNF-α might contribute to the LDAEP increase seen after ECT. Given these multifaceted biological interactions, further research is indeed warranted to unravel the complexities of ECT’s impact on the LDAEP and underlying neurobiological mechanisms.
Moreover, the primary auditory cortex is located within the superior temporal gyrus (STG). Significant changes in the LDAEP likely indicate changes in neural activity within parts of the STG anatomically and functionally connected to the PAC and potentially within the temporal lobe in general. For example, in a 2020 study, Pillai et al. found that the LDAEP was significantly correlated positively with the 5-HT1a receptor and negatively with 5-HTT throughout the temporal cortex (15). In the case of an altered LDAEP, far-reaching effects within the brain are likely given the temporal cortex’s pivotal role in the default mode network, social cognition network, and executive control network. Future work can employ techniques such as dynamic causal modeling to estimate how different brain regions interact during the processing of the auditory stimuli, thus allowing for more insightful delineation of the specific neural pathways involved in LDAEP.
In terms of cognitive performance, our study found that the pre-ECT LDAEP correlated with baseline RBANS total scores. However, change in LDAEP was not associated with changes in cognitive performance post-ECT. While the reasons for these findings warrant further investigation, our preliminary data does suggest a link between LDAEP and cognitive performance metrics.
Finally, it is crucial to acknowledge the limitations inherent in this pilot study. The small sample size of nine participants may not fully represent the broader patient population; these findings must be regarded as exploratory. It is important to recognize that the participants in this study are older adults, with six participants aged 65 or older. Age can influence clinical responses and ECT-induced biomarker changes. However, this study does not have the statistical power to assess the influence of age adequately. Future studies with larger and more diverse samples are needed to confirm our findings and better understand the impact of age and sex on ECT treatment efficacy and related biomarkers. Furthermore, the impact of the patients’ ongoing psychotropic medication on the LDAEP results also cannot be overlooked. The heterogeneous treatment responses and age can also influence the LDAEP outcome. Despite these limitations, this study provides meaningful insights into the changes in LDAEP following ECT and signals the importance of conducting larger-scale, more controlled research to elucidate these preliminary observations.
5 ConclusionContrary to our initial hypothesis, ECT paradoxically led to an increase in LDAEP, implying a reduction in serotonergic activity. Given the complex roles ECT plays in the brain’s neurochemistry and the multi-faceted nature of LDAEP as a biological marker, our results might not signal a straightforward suppression of serotonin. They could reflect compensatory adjustments in serotonergic receptor activity or broader changes encompassing other neurotransmitter systems, neuroplasticity, and neuro-immune interactions. This unexpected outcome opens avenues for multiple lines of inquiry: the intricate interplay between ECT and LDAEP, and how they might influence 1) the activity of serotonergic, dopaminergic, glutamatergic, and GABAergic receptors and transporters; 2) neuroplasticity and BDNF levels in the temporal cortex; and 3) levels of the pro-inflammatory cytokine TNF-α. Looking forward, further investigation is needed to validate the LDAEP as a biomarker of serotonergic neurotransmission and to elucidate ECT’s effect on serotonergic activity in the human brain.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by University of New Mexico (UNM) Human Research Protections Office. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsMD: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. JL: Methodology, Validation, Writing – review & editing. CA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Z-DD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from the National Institute of Mental Health (MH128692 and MH125126, Abbott). Z-DD is supported by the National Institute of Mental Health Intramural Research Program (ZIAMH002955).
Conflict of interestZ-DD is inventor on patents and patent applications on electrical and magnetic brain stimulation therapy systems held by the National Institutes of Health NIH, Columbia University, and University of New Mexico. CA is inventor on patents and patent applications on electrical brain stimulation therapy systems held by University of New Mexico.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes Note that “LDAEP” traditionally refers to potentials recorded via EEG. For consistency with the literature, we retain this familiar nomenclature. However, it is important to note that in the context of this study, “LDAEP” denotes the magnetic fields measured by MEG rather than electrical potentials.References4. Deng ZD, Robins PL, Regenold W, Rohde P, Dannhauer M, Lisanby SH. How electroconvulsive therapy works in the treatment of depression: is it the seizure, the electricity, or both? Neuropsychopharmacology. (2024) 49:150–62. doi: 10.1038/s41386-023-01677-2
PubMed Abstract | Crossref Full Text | Google Scholar
6. Rojas M, Ariza D, Ortega A, Riaño Garzón ME, Chávez-Castillo M, Pérez JL, et al. Electroconvulsive therapy in psychiatric disorders: A narrative review exploring neuroendocrine-immune therapeutic mechanisms and clinical implications. Int J Mol Sci. (2022) 23:6918. doi: 10.3390/ijms23136918
PubMed Abstract | Crossref Full Text | Google Scholar
7. Ousdal OT, Brancati GE, Kessler U, Erchinger V, Dale AM, Abbott C, et al. The neurobiological effects of electroconvulsive therapy studied through magnetic resonance: What have we learned, and where do we go? Biol Psychiatry. (2022) 91:540–9. doi: 10.1016/j.biopsych.2021.05.023
PubMed Abstract | Crossref Full Text | Google Scholar
8. Maffioletti E, Carvalho Silva R, Bortolomasi M, Baune BT, Gennarelli M, Minelli A. Molecular biomarkers of electroconvulsive therapy effects and clinical response: Understanding the present to shape the future. Brain Sci. (2021) 11:1120. doi: 10.3390/brainsci11091120
PubMed Abstract | Crossref Full Text | Google Scholar
9. Belge JB, Mulders P, Van Diermen L, Sienaert P, Sabbe B, Abbott CC, et al. Reviewing the neurobiology of electroconvulsive therapy on a micro-, meso-, and macro-level. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 127:110809. doi: 10.1016/j.pnpbp.2023.110809
PubMed Abstract | Crossref Full Text | Google Scholar
10. Cano M, Camprodon JA. Understanding the mechanisms of action of electroconvulsive therapy: Revisiting neuroinflammatory and neuroplasticity hypotheses. JAMA Psychiatry. (2023) 80:643–4. doi: 10.1001/jamapsychiatry.2023.0728
PubMed Abstract | Crossref Full Text | Google Scholar
13. Juckel G, Hegerl U, Molnár M, Csépe V, Karmos G. Auditory-evoked potentials as indicator of brain serotonergic activity–first evidence in behaving cats. Biol Psychiatry. (1997) 41:1181–95. doi: 10.1016/s0006-3223(96)00240-5
PubMed Abstract | Crossref Full Text | Google Scholar
14. Lewis DA, Campbell MJ, Foote SL, Morrison JH. The monoaminergic innervation of primate neocortex. Hum Neurobiol. (1986) 5:181–8.
PubMed Abstract | Google Scholar
15. Pillai R, Bartlett E, Ananth M, Zhu Z, Yang J, Hajcak G. Examining the underpinnings of loudness dependence of auditory evoked potentials with positron emission tomography. Neuroimage. (2020) 213:116733. doi: 10.1016/j.neuroimage.2020.116733
PubMed Abstract | Crossref Full Text | Google Scholar
16. Juckel G, Hegerl U, Molnár M, Csépe V, Karmos G. Auditory evoked potentials reflect serotonergic neuronal activity–a study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology. (1999) 21:710–6. doi: 10.1016/S0893-133X(99)00074-3
PubMed Abstract | Crossref Full Text | Google Scholar
18. Manjarrez G, Hernandez E, Robles A, Hernandez J. N1/P2 component of auditory evoked potential reflect changes of the brain serotonin biosynthesis in rats. Nutr Neurosci. (2005) 8:213–8. doi: 10.1080/10284150500170971
PubMed Abstract | Crossref Full Text | Google Scholar
19. Hegerl U, Gallinat J, Juckel G. Event-related potentials. do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord. (2001) 62:93–100. doi: 10.1016/s0165-0327(00)00353-0
PubMed Abstract | Crossref Full Text | Google Scholar
20. Kangas ES, Vuoriainen E, Lindeman S, Astikainen P. Auditory event-related potentials in separating patients with depressive disorders and non-depressed controls: A narrative review. Int J Psychophysiol. (2022) 179:119–42. doi: 10.1016/j.ijpsycho.2022.07.003
PubMed Abstract | Crossref Full Text | Google Scholar
21. Roser P, Kawohl W, Juckel G. Chapter 20 - The loudness dependence of auditory evoked potentials as an electrophysiological marker of central serotonergic neurotransmission: Implications for clinical psychiatry and psychopharmacotherapy (Elsevier). In: Handbook of behavioral neuroscience, vol. 31 (Cambridge, MA: Academic Press), (2020). p. 361–74. doi: 10.1016/B978-0-444-64125-0.00020-7
Crossref Full Text | Google Scholar
22. Pan W, Pan J, Zhao Y, Zhang H, Tang J. Serotonin transporter defect disturbs structure and function of the auditory cortex in mice. Front Neurosci. (2021) 15:749923. doi: 10.3389/fnins.2021.749923
PubMed Abstract | Crossref Full Text | Google Scholar
23. Rao D, Basura GJ, Roche J, Daniels S, Mancilla JG, Manis PB. Hearing loss alters serotonergic modulation of intrinsic excitability in auditory cortex. J Neurophysiol. (2010) 104:2693–703. doi: 10.1152/jn.01092.2009
PubMed Abstract | Crossref Full Text | Google Scholar
24. Lee KKY, Soutar CN, Dringenberg HC. Gating of long-term potentiation (LTP) in the thalamocortical auditory system of rats by serotonergic (5-HT) receptors. Brain Res. (2018) 1683:1–11. doi: 10.1016/j.brainres.2018.01.004
PubMed Abstract | Crossref Full Text | Google Scholar
25. Steinberg LJ, Underwood MD, Bakalian MJ, Kassir SA, Mann JJ, Arango V. 5-HT1A receptor, 5-HT2A receptor and serotonin transporter binding in the human auditory cortex in depression. J Psychiatry Neurosci. (2019) 44:294–302. doi: 10.1503/jpn.180190
PubMed Abstract | Crossref Full Text | Google Scholar
26. O’Neill BV, Croft RJ, Nathan PJ. The loudness dependence of the auditory evoked potential (LDAEP) as an in vivo biomarker of central serotonergic function in humans: Rationale, evaluation and review of findings. Hum Psychopharmacol. (2008) 23:355–70. doi: 10.1002/hup.940
PubMed Abstract | Crossref Full Text | Google Scholar
29. Tang ZQ, Trussell LO. Serotonergic modulation of sensory representation in a central multisensory circuit is pathway specific. Cell Rep. (2017) 20:1844–54. doi: 10.1016/j.celrep.2017.07.079
PubMed Abstract | Crossref Full Text | Google Scholar
31. Christ M, Michael N, Hihn H, Schüttke C, Konrad C, Baune BT, et al. Auditory processing of sine tones before, during and after ECT in depressed patients by fMRI. J Neural Transm. (2008) 115:1199–211. doi: 10.1007/s00702-008-0036-5
PubMed Abstract | Crossref Full Text | Google Scholar
32. Zwanzger P, Zavorotnyy M, Diemer J, Ruland T, Domschke K, Christ M, et al. Auditory processing in remitted major depression: A long-term follow-up investigation using 3T-fMRI. J Neural Transm. (2012) 119:1565–73. doi: 10.1007/s00702-012-0871-2
PubMed Abstract | Crossref Full Text | Google Scholar
33. Zweerings J, Zvyagintsev M, Turetsky BI, Klasen M, König AA, Roecher E, et al. Fronto-parietal and temporal brain dysfunction in depression: A fMRI investigation of auditory mismatch processing. Hum Brain Mapp. (2019) 40:3657–68. doi: 10.1002/hbm.24623
PubMed Abstract | Crossref Full Text | Google Scholar
34. Opitz B, Schröger E, von Cramon DY. Sensory and cognitive mechanisms for preattentive change detection in auditory cortex. Eur J Neurosci. (2005) 21:531–5. doi: 10.1111/j.1460-9568.2005.03839.x
PubMed Abstract | Crossref Full Text | Google Scholar
35. Cervantes-Ramírez V, Canto-Bustos M, Aguilar-Magaña D, Pérez-Padilla EA, Góngora-Alfaro JL, Pineda JC, et al. Citalopram reduces glutamatergic synaptic transmission in the auditory cortex via activation of 5-HT1A receptors. Neuroreport. (2019) 30:1316–22. doi: 10.1097/WNR.0000000000001366
PubMed Abstract | Crossref Full Text | Google Scholar
36. Timmermann C, Spriggs MJ, Kaelen M, Leech R, Nutt DJ, Moran RJ, et al. LSD modulates effective connectivity and neural adaptation mechanisms in an auditory oddball paradigm. Neuropharmacology. (2018) 142:251–62. doi: 10.1016/j.neuropharm.2017.10.039
PubMed Abstract | Crossref Full Text | Google Scholar
37. Ampuero E, Cerda M, Härtel S, Rubio FJ, Massa S, Cubillos P, et al. Chronic fluoxetine treatment induces maturation-compatible changes in the dendritic arbor and in synaptic responses in the auditory cortex. Front Pharmacol. (2019) 10:804. doi: 10.3389/fphar.2019.00804
PubMed Abstract | Crossref Full Text | Google Scholar
38. Griskova I, Dapsys K, Andruskevicius S, Ruksenas O. Does electrocon
Comments (0)