The human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) pandemic over the last 40 years has modified the clinical and epidemiological spectrum of leishmaniasis. An overlap between the areas of visceral leishmaniasis (VL) transmission and HIV infection has been clearly observed, with one-third of HIV cases worldwide occurring in areas at risk of leishmaniasis transmission (1, 2). Consequently, co-infection of VL with HIV (VL/HIV) has emerged as an important challenge in VL control, with VL itself becoming an important opportunistic disease associated with HIV infection (3). Herein, we carefully explain the main immunopathogenic mechanisms underlying the association between visceral leishmaniasis and HIV infection. Moreover, we also pointed out how the knowledge generated from cross-sectional and longitudinal studies helped to predict the different clinical outcomes of these patients, particularly in terms of disease severity or relapses.
Epidemiology of VL/HIV co-infectionSince the 1980s, when the first case of leishmaniasis associated with HIV infection was published (4), an increase in the cases of co-infection has been recorded. In the 1990s, the majority of co-infection cases were reported in European countries in the Mediterranean region (Spain, France, Italy, and Portugal), where 1.5% to 9% of individuals with AIDS presented with new cases or reactivation of infection by viscerotropic species of Leishmania (5). The prevalence of VL in this population was 500 times higher than that in the non-HIV-infected population (6).
Subsequently, the advent of combined antiretroviral therapy (cART) modified this epidemiological scenario, resulting in a progressive decrease in VL/HIV cases in the Mediterranean basin and a considerably low incidence in this region (7). Nevertheless, 45 countries worldwide have reported cases of Leishmania/HIV co-infection, with the visceral form being the most prevalent (3). The most critical incidence scenario has been reported in some African countries, such as Sudan and Ethiopia, where 35% of individuals with VL have HIV co-infection, as well as in the state of Bihar in India and the countries in Central and South America, particularly Brazil.
Considering the elevated number of cases of both infections in Brazil, it is also expected to have the highest incidence of VL/HIV co-infection in the American continent. This epidemiological profile can be attributed to the spread of HIV infection to rural areas, urbanization of the VL vector, or even the evident urban problem of sharing of contaminated needles by drug users (8). In addition, some factors related to diagnosis may also be involved in this highest incidence. Firstly, the greater predisposition to symptomatic VL between immunosuppressed by HIV infection can lead to the opening of a VL case, or even to the reactivation of a latent infection. Moreover, the occurrence of VL/HIV in urban areas often facilitates the search for medical consultations and a faster parasitological diagnosis. Finally, it is believed that the computerized health notification system, together with the existence of databases that allow cross-referencing between them, can allow for better monitoring and notification by surveillance system. In this way, since 2019, the prevalence of VL/HIV co-infection has progressively increased, reaching >11% (9), which may still be underreported due to a reduction in the number of confirmed cases of VL during the coronavirus disease 2019 pandemic. Therefore, although the prevalence has increased, it is important to consider that these percentages only reflect cases showing clinical manifestations of VL; asymptomatic cases may be diagnosed late, and a significant number of patients with VL do not undergo serological investigation for HIV (10), indicating that the actual scenario is even more worrying.
In 2023, approximately 300 new cases of VL/HIV co-infection were reported to the Brazilian Ministry of Health, accounting for approximately 19% of the VL cases reported during this period (9). In line with the simultaneous expansion and geographic overlap of both infections, most cases of VL/HIV co-infection in Brazil have been reported in the Northeast, Midwest, and Southeast regions, especially in the states of Maranhão, Mato Grosso do Sul, and Minas Gerais, respectively (9). Young adult males aged 20-49 years and injection drug users are the most affected groups, representing an ever-expanding exposure category (11). Additionally, in 41% of the cases, the diagnosis of both infections occurred simultaneously, with VL being responsible for opening up an HIV/AIDS case (11). In contrast, latent VL has also been reported in HIV-infected individuals, who show a higher risk of VL relapse, especially when CD4+ T-lymphocyte absolute counts reach levels < 200 cells/mm3, making them possible reservoirs for the parasite (12).
Then, one important factor is that Leishmania and HIV infection reinforce each other, posing significant clinical and public health problems. It is worth noting that important socio-demographic issues also permeate this association at Brazil. Social vulnerability places VL/HIV patients in conditions of malnutrition and exposure to factors that may favor the development of symptomatic VL. On the other hand, patients who commonly come from rural areas experience geographical limitations and mobility difficulties that can make difficult their access to effective treatment and clinical monitoring offered at reference centers in urban areas. Therefore, providing strategies that go beyond the currently recommended protocols, particularly with regard to predicting prognosis, can contribute to long-term clinical improvement, resulting in quality of life for the patient and lower costs for the healthcare system.
Evidence for VL as an HIV/AIDS-related diseaseAlthough it is not yet considered an AIDS-defining disease, VL may contribute to the immunosuppression and other immunological disturbances associated with rapid progression to AIDS (13–16). However, HIV co-infection has been shown to accelerate the development of active VL and reduce the probability of a successful response to leishmanial therapy, increasing the VL lethality rate and enhancing the predisposition to VL relapse by 3-5 times in comparison with that in HIV-negative individuals (10, 12, 17). In this context, Leishmania/HIV co-infection represents a major challenge for management of VL, since both infections share immunopathogenic characteristics that can reciprocally affect co-infected patients.
VL immunopathogenesis is characterized by systemic involvement, since the amastigote forms of L. (L.) infantum and L. (L.) donovani show marked tropism for mononuclear phagocytic cells of the spleen, liver, bone marrow, and lymph nodes. This leads to impaired host immune defense mechanisms because of a shift in the production of myeloid and lymphoid cells in the bone marrow as a result of intense parasitism and destruction of mononuclear cells and the consequent attempts to replace them. In laboratory assessments, classic pancytopenia is characterized mainly by neutropenia, lymphopenia, anemia, and thrombocytopenia (18–21). Lymphopenia may be caused by deviations in the production of T-lymphocyte progenitors and by thymic atrophy as a result of undernutrition and parasitism (22–24). Additionally, activation-induced cell death in the periphery may be associated with lymphopenia. More recently, CD4+ T-cell depletion has been suggested to be caused by pathogenic changes in the spleen due to advanced white pulp disorganization and splenic depletion of CD4+ T cells by apoptosis and pyroptosis secondary to HIV and parasite infection (25). Independent of the mechanisms involved, all of them can contribute to the impairment of a specific immune response against the parasite, characterizing VL as an immunosuppressive disease (Figure 1).
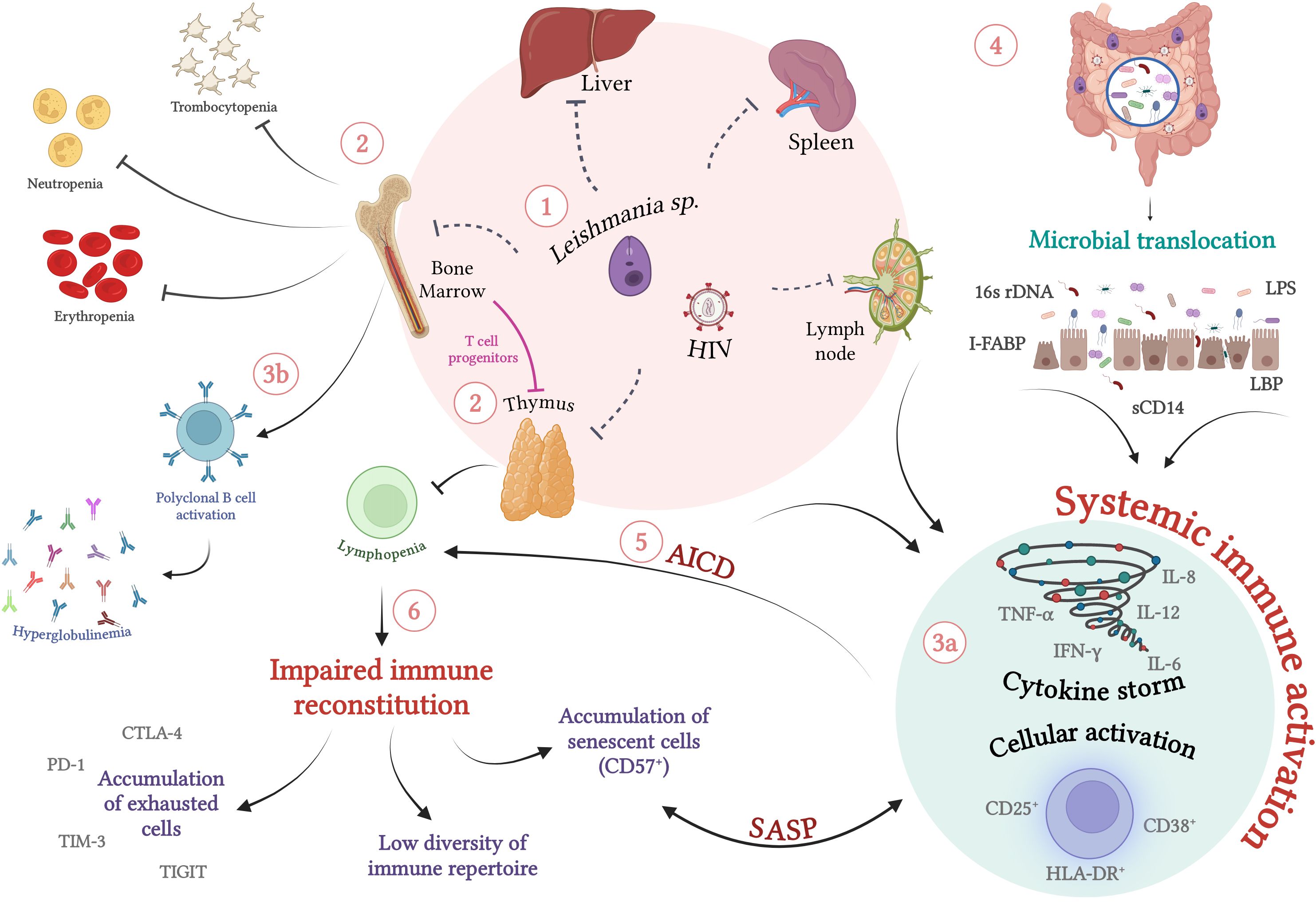
Figure 1. Immunopathogenesis of visceral leishmaniasis in HIV-infected individuals: Chronic immune activation and its implications for the immune impairment of VL/HIV co-infection (1) Co-infection with L. infantum/L. donovani and HIV is characterized by simultaneous systemic involvement of organs such as lymph nodes and thymus, in addition to parasitism of the bone marrow, spleen and liver (2). The intense parasitism of monocytes and macrophages by the protozoan culminates in changes in the production of myeloid and lymphoid progenitors by the bone marrow. In an attempt to recover the parasitized cells, there is a shift in favor of this lineage, which can lead to a laboratorial profile of erythropenia, neutropenia, thrombocytopenia and lymphopenia. In VL/HIV co-infection, the drop in absolute counts of peripheral CD4 T lymphocytes is enhanced by infection and concomitant thymic impairment by HIV and the protozoan. (3a) In parallel with immunosuppression, co-infection occurs with high cellular activation, characterized by activated T lymphocytes, high production of inflammatory cytokines (cytokine storm) and (3b) polyclonal activation of B lymphocytes, which culminates in hyperglobulinemia (4). In addition to parasite and viral antigens, the translocation of microbial products has been identified as an important cofactor for increasing levels of systemic activation (5). As a result of this persistent activation, activation-induced cell death (AICD), worsening of lymphopenia and an impairment of the cellular immune response specific to the parasite is observed, which may favor relapses (6). The maintenance of this scenario can result in the exhaustion of primary immune resources (thymus and bone marrow) and the inability of lymphocytic repopulation. This process results in the deterioration of the responsive capacity to parasitic stimuli, favoring the accumulation of exhausted and/or terminally differentiated cells and the low diversity of the lymphocyte repertoire. SASP, Senescence-Associated Secretory Phenotype. Dashed lines indicate direct damage caused by pathogens. Source: The author. Created with BioRender.com.
Indeed, the evolution of active VL is characterized by specific immunosuppression for parasite antigens since the delayed-type hypersensitivity test (Montenegro test) shows negative results, unlike in individuals with asymptomatic or subclinical disease (26–28). In addition, patients with active VL show a decrease in the proliferative capacity of helper T cells when stimulated in vitro with antigens of the parasite and also a decrease in the production of specific cytokines such as interleukin (IL)-2 and interferon (IFN)-γ, alongside an increase of IL-10 (26, 27, 29, 30).
Absolute CD4+ T-cell counts play a key role in the prognosis of VL in terms of immunosuppression and immunological reconstitution. Patients with VL show lower CD4+ T-cell counts than healthy individuals. Although the CD4+ T-cell counts recover during the remission phase, they do not reach normal levels (31). A recent study of our group showed that this CD4+ T-cell depletion is associated with the clinical prognosis. Patients with non-relapsed VL showed a significant increase in the number of these cells shortly after treatment, which was not observed in patients with relapse (32). Although this evidence indicates the importance of evaluating this parameter in clinical practice, it is not routinely assessed in patients with non-HIV VL.
Paradoxically, VL immunopathogenesis evolves with an intense degree of activation of the immune system. Our group demonstrated that patients with active VL present high percentages of activated T lymphocytes, which remain elevated even after clinical remission (31). However, when these cells were stimulated with Leishmania antigens, the percentage of activated cells was lower than that of lymphocytes from individuals in clinical remission, corroborating the specific immunosuppressive profile of the active phase of VL (33). Additionally, other studies have suggested that VL is characterized by an exacerbated systemic inflammatory response mediated by inflammatory cytokines such as IL-8, tumor necrosis factor (TNF), and IL-6, especially in active disease (34, 35). This inflammatory and activated status has been associated with multiple organ failure, showing similarities with the findings observed in sepsis, malaria, and other inflammatory diseases (36, 37).
Similar immunopathogenic aspects have been observed in patients with HIV infection. CD4+ T lymphocytes play a central role in the acquired immune responses against HIV. As the infection progresses, the continuous loss of these cells, especially to levels below 200 cells/mm3, favors opportunistic infections such as tuberculosis or VL (38, 39).
The persistence of the anti-HIV immune response, in addition to factors that go beyond viral antigens, leads to chronic immune activation (40–42). High levels of pro-inflammatory cytokines, such as TNF, IL-6, and IL-1β, and chemokines, such as regulated on activation, normal T-cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α, MIP-1β, and chemokine ligand 13 (CXCL13) have also been directly implicated in this process (43–47).
Since Leishmania sp. and HIV infect the same target cells and thus compromise the same immune compartments, they can be reasonably expected to cause reciprocal impairment of the effector immune response and, consequently, of the control of each pathogen (48). The immunological alterations observed in VL and HIV/AIDS can synergistically affect co-infected patients. Therefore, one of the most critical hallmarks of VL/HIV co-infection is severe immunosuppression and intense chronic immune activation (Figure 1), especially that caused by a cytokine storm, such that VL results in worsening of the clinical condition of patients co-infected with HIV.
Indeed, the chronic stimulation observed in VL can increase viral replication and latent provirus expression, resulting in faster progression to AIDS (49). Leishmania spp. can augment viral replication by inducing cellular activation and an inflammatory microenvironment, which in turn increases the susceptibility of target cells to infection (50–52). Indeed, the lipophosphoglycan (LPG) molecule presenting on the parasite’s surface activates the transcription factor nuclear factor (NF)-ƙB, increasing TNF production (53, 54). However, the role of LPG in the Leishmania-HIV interaction is still controversial because LPG also inhibits viral entry into monocyte-derived macrophages (MDMs) in the early phase of infection, resulting in reduced viral replication (55).
L. infantum-infected human dendritic cells co-cultured with autologous CD4+ T lymphocytes show increased viral replication, probably through the secretion of cytokines such as IL-6 and TNF (51). Accordingly, VL/HIV co-infected patients present with high levels of TNF and IL-6, even with the use of cART and anti-Leishmania treatment, which may be associated with high viremia and progressive loss of CD4+ T lymphocytes (56–58).
In addition to influencing the viral load of patients co-infected with HIV, VL can potentiate the depletion of CD4+ T lymphocytes (59). Low absolute counts of CD4+ T lymphocytes have been observed in VL/HIV-co-infected patients during the VL active phase and clinical remission despite cART (60, 61). Notably, these counts were lower than those observed in individuals with HIV mono-infection (61). Takele et al. also reported low counts of CD4+ T cells in the active phase in co-infected patients and suggested that these low counts may be associated with low production of antigen-specific IFN-γ in this phase (62), further confirming that such impairments can predispose individuals to disease recurrence. Thus, VL and its immunopathogenic consequences can aggravate the immunodeficiency caused by HIV, not only enhancing the degree of immune activation in VL/HIV co-infected patients (61), but also worsening CD4+ T-cell depletion.
Simultaneously, the HIV-induced disorganization of the immune system and the depletion of the pool of specific T lymphocytes severely compromise the mechanisms of parasite control, contributing to a higher parasite load and progression of VL. Indeed, HIV-infected patients, especially those with CD4+ T-lymphocyte counts < 200 cells/mm3, are at a high risk of progression to symptomatic VL (12, 15, 58, 60, 63, 64). In this scenario, VL/HIV patients are more susceptible to the development of unusual clinical manifestations and/or dissemination of VL to atypical sites (skin, gastrointestinal tract, adrenal glands, cerebrospinal fluid, respiratory tract, cardiac and renal tissues, etc.) (65–67).
Furthermore, although clinical remission of VL is commonly achieved after anti-Leishmania treatment, parasitemia appears to persist, even if intermittently (50). This leads to a condition called “chronically active VL,” which is associated with immunological impairment and may explain the higher susceptibility of patients with VL/HIV co-infection to multiple VL relapses (12). Additionally, the greater treatment resistance and more frequent occurrence of therapeutic failure in these patients may be related to parasitic persistence after specific therapies (49). Thus, although both infections share similar immunological characteristics, co-infected patients may not present the same clinical and laboratory profiles as HIV or Leishmania-infected individuals.
Post-cART immune reconstitution in patients with VL/HIV co-infectionAlthough cART has undoubtedly contributed to reducing the incidence of opportunistic infections in HIV patients, it does not seem to restore the immune response in patients with VL/HIV co-infection. Indeed, antiretrovirals from the protease inhibitor (PI) class have shown direct inhibitory effects on the evolutionary forms of L. (L.) major, L. (L.) amazonensis, L. (V.) braziliensis and L. (L.) infantum in vitro (49, 68). Moreover, the leishmanicidal activity observed in L. major promastigote forms has been attributed to proteasome inhibition (69). Also, PIs appear to inhibit the replication of promastigote forms, the proliferation of amastigotes within macrophages and prevent the development of lesions in infected mice (70). Interestingly, when an L. infantum strain was isolated from co-infected patients undergoing cART, no inhibitory effect of PIs was observed (71). Thus, while the beneficial effects of cART on the virus and, experimentally, on the parasite are indisputable, co-infected patients present VL relapse, suggesting that immune response impairment plays a key role in the clinical prognosis.
Unlike other opportunistic diseases associated with HIV, post-cART immune reconstitution is still severely impaired in VL/HIV co-infection, as shown by low CD4+ T-cell counts even in patients with an undetectable HIV viral load and clinical remission after treatment of VL (49, 60, 61).
This persistent immunosuppression in patients with VL/HIV co-infection corroborates the fact that few studies have reported the occurrence of VL in association with immune reconstitution inflammatory syndrome (IRIS), which is most frequently observed in post-kala-azar dermal leishmaniasis (PKDL) (72–75), followed by tegumentary leishmaniasis and uveitis (76, 77). IRIS is characterized by a transient, but sometimes severe, local and systemic inflammatory response directed against a known condition (e.g., opportunistic pathogens or autoimmune diseases) in HIV-infected patients shortly after cART initiation due to the improvement in CD4+ T-cell counts (78). The primary treatment of VL in cases of concomitant HIV diagnosis preceding cART (11) should reduce the available antigenic load to stimulate the pool of specific cells post-cART reconstitution, thereby reducing the risk of IRIS, similar to the findings observed in tuberculosis/HIV co-infection (79, 80).
In the context of VL relapse, the CD4+ T-lymphocyte count is one of the most common parameters for predicting the clinical evolution of patients with VL/HIV co-infection (12). Through prospective follow-up, we demonstrated that co-infected patients who developed several episodes of VL showed low CD4+ T-lymphocyte counts for up to 12 months after treatment, while patients presenting with a single episode of VL showed a significant gain in these cells after anti-Leishmania treatment (15). Interestingly, both relapsed and non-relapsed groups were undergoing cART and showed viral suppression (15), suggesting that the cART does not seem to be able to prevent frequent relapses, especially the visceral form of the disease (12).
In this scenario, the maintenance of anti-Leishmania treatment using secondary prophylaxis could hypothetically be effective in reducing disease relapse in patients with VL/HIV co-infection (12, 81). Therefore, after specific anti-Leishmania therapy, these patients remained on a prophylactic regimen to avoid new active episodes of the disease. According to the Brazilian Ministry of Health, secondary prophylaxis should be administered when a patient with VL/HIV co-infection shows absolute CD4⁺ T-lymphocyte counts lower than 350 cells/mm3, and commonly involves administration of liposomal amphotericin B at a dose of 3–5 mg/kg every two weeks (11).
However, in most cases of VL/HIV co-infection, these specific therapeutic regimens control the parasite load in the peripheral blood within a short time period, and patients still show recurrence of active disease (82–84). A recent study conducted in a Brazilian referral hospital found that VL relapses occurred in 36.4% of the patients with VL/HIV co-infection receiving secondary prophylaxis (85). The absence of immune reconstitution in relapsed patients was noted even in patients receiving secondary prophylaxis (15). Thus, other factors, in addition to the virus and the parasite itself, could contribute to the poor clinical prognosis of patients with VL/HIV co-infection. Finally, additional studies are necessary to confirm the effectiveness of secondary prophylaxis in severely immunocompromised patients.
Immune activation and inflammatory status in patients with VL/HIV co-infectionIn patients with VL/HIV co-infection, chronic immune activation and severe immunosuppression may be potentiated (13, 58, 61, 86), which is an important cofactor in immunological impairment (15, 16, 87). In addition to favoring VL relapse, the degree of immune activation and inflammatory status may be associated with the occurrence of atypical manifestations of VL, such as cutaneous dissemination or PKDL (67, 88), or even progression to severe VL in co-infected patients (58) as well as in patients without HIV infection (37).
We had shown for the first time that patients with VL/HIV co-infection in VL clinical remission present with high percentages of CD8+ T cells expressing CD38 (61). Furthermore, these activated T cells were associated with low counts of CD4+ T lymphocytes regardless of the use of cART, undetectable viral loads, or clinical remission of VL due to anti-Leishmania treatment (61). This cellular activation status was later confirmed in patients with VL/HIV co-infection in the active phase of VL, when patients already had a low or undetectable parasite load combined with effective viral control (13). Subsequently, other studies confirmed high percentages of activated CD8+ T lymphocytes (CD38+HLA-DR+) in Brazilian or Spanish co-infected patients with asymptomatic VL (86) or previous history of VL relapses (87), respectively.
The presence of high serum cytokine levels has also been investigated as a predictor of the clinical progression of VL (37, 58, 89–91). Indeed, similar to T-cell activation, the plasma levels of pro-inflammatory cytokines (IFN-γ, IL-6, IL-8, TNF, and MIP-1β) were higher in patients with VL/HIV co-infection than in those with HIV or VL mono-infection and healthy individuals (13). More recently, IFN-γ and TNF levels have been correlated with severity and death as well as clinical findings such as vomiting and dyspnea in patients with VL/HIV co-infection (58).
In this scenario, long-term follow-up of one patient with VL/HIV co-infection who presented with VL relapse with cutaneous manifestations three months post-therapy showed that this episode was associated with an increase in T-cell activation (67). These data provide the first evidence of a relationship between immune activation and VL relapse. Subsequently, in the cohort evaluated up to 12 months post-treatment, patients with non-relapsing and relapsing VL/HIV showed similar levels of activation of CD4+ and CD8+ T cells (CD38+HLA-DR+ expression) in the active phase of VL, but only those with non-relapsing VL/HIV showed a long-term reduction in these percentages post-treatment (15). Patients with relapsing VL/HIV also showed persistently higher levels of pro-inflammatory cytokines (IL-8, TNF, IFN-γ, IL-6, IL-1β, and others) and IL-10 than those with non-relapsing VL/HIV, who tended to show gradual reductions in the levels of these cytokines after treatment (16).
Since patients with relapsing VL/HIV show a high inflammatory status even under effective anti-Leishmania treatment and cART, L. infantum infection can be plausibly considered to not be the sole factor responsible for enhancing immune activation in HIV-infected individuals.
Microbial translocation as an additional factor influencing immune activation in VL/HIV co-infectionMicrobial translocation from the intestinal lumen to the bloodstream, a phenomenon known to occur in HIV infection (92–94) and already evidenced in VL (31, 90), could constitute another important cofactor for maintaining a high degree of activation in VL/HIV patients (Figure 1). In HIV or simian immunodeficiency virus (SIV) infections, damage to the intestinal barrier, characterized by the death of enterocytes resulting in increased permeability, can be mediated by viral replication itself. Indirect mechanisms such as massive destruction of memory CD4+CCR5+ T cells present in the intestinal mucosa and/or loss of IL-17-producing cells may also contribute to this damage, since they are crucial in the response to bacterial antigens through the neutrophil infiltration, and maintenance of intestinal homeostasis (92, 93, 95–99). As a result, HIV-infected patients present symptoms characteristic of enteropathy, such as diarrhea, malabsorption, inflammatory infiltrates, villus atrophy, and crypt hyperplasia in the mucous tissue (100). Thus, this evidence favors the translocation of bacterial products into systemic circulation (100), constituting one of the main immunopathogenic mechanisms associated with HIV infection (93, 101, 102).
The following molecules have become hallmarks of chronic immune stimulation due to microbial translocation: intestinal fatty acid binding protein (I-FABP) (103), lipopolysaccharide (LPS) from gram-negative bacteria, and soluble receptor CD14 (sCD14) (92, 104, 105). Bacterial components stimulate the cells involved in innate immunity through Toll-like receptor (TLR) ligands (106). LPS, for example, binds to its receptor CD14 and the TLR4-MD2 complex, culminating in the activation of the transcriptional factor NF-κB and the production of inflammatory cytokines such as IL-6, IL-1β, TNF, and IFN type-I. As mentioned previously, these cytokines contribute to the persistent local and systemic immune activation observed during the chronic phase of HIV infection, resulting in a vicious circle. Moreover, elevated LPS levels have been linked to high indices of immune activation in CD8+ T cells in HIV-infected patients (92, 104, 107).
Amastigote forms observed in the mucosa-associated lymphoid tissue (MALT) of patients with VL (108, 109) can lead to intestinal damage. Translocation of microbial products, which is implied by increased plasma levels of LPS, its sCD14 receptor, and I-FABP, has been observed in patients with active VL (31). Such elevated LPS levels are correlated with T-cell activation and high levels of pro-inflammatory cytokines such as macrophage migration inhibitory factor (MIF) and IL-8 (31). In patients with VL/HIV, LPS levels are positively correlated with sCD14 levels, specifically in patients with low CD4+ counts (<200 cells/mL) (58), as well as with the percentages of activated CD8+ T lymphocytes and IL-6 and IL-8 levels (13). The levels of these molecules have also been shown to be significantly increased in patients with previous VL in comparison with those showing an immunodiscordant response to cART (IDR; CD4 count < 200 cells/μL) without VL (87). In this context, a recent study showed that sCD14 is the only independent predictor of disease severity and death in VL/HIV co-infection (58). Furthermore, the levels of other soluble factors associated with microbial translocation and intestinal damage, such as MIF and I-FABP, have also been shown to be elevated in patients with VL/HIV, both in the active phase and in remission (13).
In terms of relapse, high levels of sCD14 were found in relapsing VL/HIV patients even at 12 months post-treatment, while the levels in non-relapsing patients decreased immediately after treatment (15). Interestingly, sCD14 levels negatively correlated with the absolute counts of CD4+ T cells, corroborating the profile of T-cell activation (CD38+HLA-DR+) in these groups (15).
This phenomenon was recently demonstrated in an elegant VL experimental model study (110), in which intestinal dysbiosis was induced in mice and hamsters by long-term treatment with broad-spectrum antibiotics (110). Weight loss, splenomegaly, and hepatomegaly were significantly less severe in the antibiotic-treated infected hamsters than in untreated animals (110), suggesting that pathobionts contribute to disease progression. Using a different approach, our group observed that infected golden hamsters show intestinal changes and evidence of bacterial translocation by increased plasma levels of LPS (111). Moreover, we verified that in comparison with untreated infected animals, infected animals treated with antimonial and amikacin showed significant reductions in the levels of LPS and activated CD4+CD25+ T cells at 60 days post-infection (dpi), as well as an increase in the percentage of CD4+ T cells at 120 dpi (111). These results provide empirical evidence for the benefits of combining VL treatment with antibiotics in affected patients.
These findings also reinforce the idea that microbial translocation can contribute to the maintenance of an intense degree of cellular activation and pro-inflammatory response, and can, therefore, directly influence the impairment of the immune response necessary for parasite control (112) (Figure 1). In other words, microbial translocation may be an additional mechanism associated with clinical outcomes such as the severity and relapse of VL in patients co-infected with HIV.
Several mechanisms underlying the activated immune status may directly affect the effector function of T lymphocytes, either quantitatively or qualitatively. Thus, similar to the enhanced degree of activation in a VL/HIV association scenario, the immunological consequences of this process may also be intensified. This hypothesis is based on the fact that each infection commonly involves a process of chronic failure of the immune system, which has been well-characterized in HIV infection and more recently in VL.
Immunological consequences of chronic immune activation and its influence on the immunopathogenesis of VL/HIV co-infectionThe deterioration of immune competence that occurs with aging is a natural process that results from successive moments of cellular activation. This process partially explains the increased morbidity and mortality among elderly individuals without pathological immunodeficiencies (113). Similarly, the intense immune activation in HIV infection has been shown to result in faster progression to immunological aging (114). Consequently, these individuals exhibit severe and early immunological impairments that usually manifest only in the elderly population (114). This process, called immunosenescence, can be clonal, with the functional loss of virus-specific clones, and/or global, with the exhaustion of central immune compartments such as the thymus and bone marrow (115).
Pro-inflammatory cytokines, such as TNF, IL-1β and IL-6, are secreted in response to various infections and tissue damage, and their secretion constitutes a complex initial cascade associated with pathogen destruction and tissue repair, which acts as the natural response to these stressful situations. However, in patients with VL or HIV infection, excessive production and/or accumulation of these mediators results in severe immunological damage. This process, which is known as inflammaging, is characterized by the hyperregulation of anti-stress responses and the production of pro-inflammatory cytokines (113). The combination of inflammaging with immunosenescence has been described to aggravate the degree of immunodeficiency in HIV infection (116, 117).
Immunosenescence is characterized by the presence of numerous clones of terminally differentiated CD4+ and CD8+ T cells (118). CD57+ T cells (118–120) and telomere shortening (114, 118) are widely used to define replicative senescence. In addition to the loss of replicative capacity, CD57+ T cells exhibit increased susceptibility to activation-induced cell death (119, 121). Depending on the specificity lost as a consequence of HIV infection (114–116, 122–125), patients may show loss of viral load control, faster progression to AIDS, and impairment of the immune response to other pathogens, such as Leishmania spp., which would be critical in the context of VL/HIV co-infection.
VL/HIV patients with and without active disease show higher percentages of senescent T cells, mainly of the CD8+ T-cell subpopulation (15, 86, 87), in comparison with patients showing HIV mono-infection, reinforcing the augmented chronically activated immune status in this association (61, 67). Despite these findings, few studies have investigated the degree of immunosenescence in VL and its consequences for the clinical outcomes of the disease.
Cellular exhaustion, which has also been explored in both infections, is phenotypically characterized by the expression of inhibitory molecules such as programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3). Both PD-1 and CTLA-4 negatively regulate T-cell activation and are characteristic markers of T-cell anergy/exhaustion during chronic infections (126). Similar to replicative senescence, but in a reversible manner, immune exhaustion results in decreased cytokine-production capacity (IL-2, TNF, and IFN-γ), as well as reduced proliferative capacity (126, 127).
Similar to HIV infection (123, 128–130), the high degree of cellular activation in VL may worsen the clinical condition of these patients by contributing to their exhaustion status. This phenomenon has been identified in human VL (126), canine VL (131, 132), and experimental VL (133) by increasing the phenotypic expression of PD-1 and CTLA-4, particularly in CD8+ T lymphocytes. In addition, specific functional impairment due to decreased proliferative capacity and IFN-Ɣ production in response to L. infantum antigens has been described (134). However, PD-1/PD-L1 pathway (PD-1 ligand) blockade can reverse this scenario, increasing the proliferative capacity against the specific antigen, restoring the Th1 response, and allowing the production of IFN-γ and increasing cytotoxic activity (133, 135). Thus, blocking these inhibitory molecules may be a promising therapeutic strategy to partially restore the immunological status of chronically infected individuals.
Although few studies have evaluated the role of these inhibitors in VL/HIV co-infection, Ethiopian VL/HIV patients, even under cART, showed high levels of T-cell immunoreceptor with Ig and ITIM domains (TIGIT) and PD1 on CD8-positive and CD8-negative T cells, along with reduced T-cell functionality as a result of the lower frequency of IFN-γ+ on TIGIT+ T cells (136). Similarly, the impaired specific production of IFN-γ seen in patients with VL/HIV co-infection may be related to the low CD4+ T-cell counts and the persistent activation/inflammation and exhaustion of T cells (33). In addition to elucidating the pathogenic mechanisms, future studies should aim to better explore the role of these molecules as possible predictors of VL severity and relapse.
The consequences of immune activation may not be limited to the loss of specific T-cell clones and phenotypic characteristics. Considering their oligoclonal and senescent state, these “terminally differentiated” cells are unlikely to be replaced by a pool of new naive T cells capable of responding to infection (137, 138). In this scenario, the exhaustion of primary immune resources is considered an important cofactor for the maintenance of this immunosenescent state in chronic infections such as HIV. Indeed, HIV-positive patients show impaired bone marrow function with loss of lymphocytic progenitors and thymic atrophy, leading to changes in immunological homeostasis and an inability to reconstitute the T and B cell compartments (139–142). This entire process can culminate in an imbalance between the specific immune response and residual viremia, resulting in the appearance and/or reappearance of other pathologies that characterize AIDS.
The thymus plays a central role in the generation of new T cells and immune reconstitution (139, 143, 144). Infection and death of thymocytes and thymic stromal cells by HIV, infection of hematopoietic stem cells, accelerated thymic atrophy, effects of pro-inflammatory cytokines (TNF), and intense degree of activation are among the factors involved in the impaired thymic function (143, 145–148). Recent thymic emigrants (RTE), newly generated T cells exported from the thymus to the periphery, can be identified by quantification of signal joint T-cell receptor (TCR) rearrangement excision circles (sjTREC) by real-time polymerase chain reaction (139). This assay involves quantification of episomal DNA that is generated during the rearrangement process of TCR genes (139) and is present mainly in cells that express TCR-αβ (Tαβ cells) (139). In addition, the CD31 molecule (PECAM-1) has also been commonly used for evaluating RTE by flow cytometry. This is because the sjTREC content is higher in naive CD4+ T cells expressing CD31, and the decline of these CD31+CD4+ T cells and TRECs levels occurs with aging (149).
Both HIV-infected humans and SIV-infected monkeys show a decrease in TREC content in naive peripheral blood T cells (139, 150–155). This decrease in thymic output is correlated with low immunological reconstitution and, consequently, low CD4+ T-cell counts (156, 157), possible loss of viremia control (158), and poor clinical prognosis in HIV infections.
However, the role of the thymus in the immunopathogenesis of VL has not yet been fully explored. Protein malnutrition in L. infantum-infected mice significantly alters the thymic microenvironment (22, 159). Atrophy, hypocellularity, and changes in the migration patterns of T-lymphocyte subpopulations were observed, in addition to reductions in the cortical area and intrathymic proliferation (22, 159). More recently, amastigote forms were found in the thymus of L. infantum-infected dogs (23).
Patients with VL may present with multifactorial injury of the T-lymphocyte lineage (1): impairment of progenitors in the bone marrow by parasitism (2), natural thymic involution that occurs with aging, and (3) thymic dysfunction induced by malnutrition, as well as the consequences of the infection itself, such as cellular activation (Figure 1).
Consequently, in the context of VL/HIV co-infection, simultaneous thymic impairment may contribute to the severity of this association. We demonstrated, for the first time, that patients with VL/HIV co-infection had lower levels of sjTRECs than those with HIV mono-infection, even under undetectable viral loads (16). Interestingly, patients with relapsing VL/HIV co-infection showed low levels of sjTRECs throughout the prospective follow-up period, whereas those with non-relapsing VL/HIV co-infection showed a significant increase at 10 months post-treatment (16), suggesting that thymic impairment may be related to the clinical outcome of VL in patients with HIV co-infection.
Disturbances in the T-lymphocyte repertoire are another qualitative consequence of immunosenescence. After thymic rearrangement, maturation, and selection, T cells migrate to the periphery. Thus, disorders of thymic function profoundly affect the diversity of the T-lymphocyte repertoire and, consequently, the capacity to respond to a variety of antigens (160). Assessments of TCR diversity are currently based on flow-cytometry and next-generation sequencing studies of the families that constitute the variable region of the β chain (Vβ). Disturbances in the Vβ repertoire have been related to the immunopathogenesis of several diseases, such as cancer (161, 162), Chagas disease (163, 164), HIV/AIDS (165–167), and leishmaniasis (16, 168, 169). Studies on American Tegumentary Leishmaniasis (ATL) showed expansion of the Vβ12 and Vβ22 families and contraction of Vβ2 in L. braziliensis-infected patients (169). Moreover, a decrease in CD8+Vβ14+ T cells in the lymph nodes has been observed (168), in contrast to the augmentation of this family in the lesions of L. guyanensis-infected patients (170), indicating the migration of these cells among immune compartments (170).
In relation to VL/HIV co-infection, unprecedentedly, our group demonstrated the occurrence of significant disorders of the Vβ repertoire (16). In this study, in comparison with healthy individuals, patients with relapsing VL/HIV co-infection showed a more heterogeneous Vβ repertoire mobilization profile throughout the follow-up period, especially in the CD8+ T cells, in terms of expansion and retraction of Vβ families. In contrast, patients with non-relapsing VL/HIV co-infection presented a profile with significant changes, although specific to certain families. The Vβ3 and Vβ18 families were less expressed in CD8+ T cells from patients with relapsing VL/HIV co-infection and expanded among patients with non-relapsing VL/HIV co-infection, especially in the active phase of the disease. Despite these findings, no characteristic profile of the dynamics of the TCRVβ repertoire in terms of clonality was observed in this study, making it impossible to associate it with clinical outcomes in terms of relapses (16).
Finally, although barely investigated, these results point to profound disturbances in the immune compartments due to associations between two parasites whose effects are progressively potentialized as a result of sharing very similar immunopathological mechanisms. Thus, prompt diagnosis and treatment of both diseases can certainly prevent more severe consequences of VL/HIV co-infection.
Relapses of VL in patients with or without HIV co-infection: potential biomarkersAccording to the Brazilian Ministry of Health, VL relapses are characterized by resurgence of symptoms within 12 months of clinical cure (11). As described by Cota et al., relapses present clinically with the reappearance of fever, worsening cytopenia, or an increase in splenomegaly after successful drug treatment (82, 171). Relapse is a very common outcome among immunosuppressed patients, such as those co-infected with HIV and transplant recipients (15, 16, 172, 173), although its incidence is also increasing among individuals without other comorbidities (32).
Horrillo et al. described treatment failures and reported that the prevalence of VL relapse was 12%, with the relapses being associated with a lack of adequate prophylaxis in patients co-infected with HIV and liposomal amphotericin B doses lower than 21 mg/kg in patients without HIV (174). Abongomera et al. described data from an Ethiopian cohort of patients with VL/HIV co-infection, in which 35% of the individuals showed relapse. cART is associated with a lower risk of relapse, whereas high parasite loads are associated with disease recurrence (175). Similarly, other studies have demonstrated that the blood parasite load (176), male sex, extremes of age (<5 and >45 years), and a slight decrease in splenomegaly (177) are risk factors for relapse in patients with VL without HIV. A recent study demonstrated that, in addition to HIV infection, factors such as thrombocytopenia, lower limb edema, and secondary pneumonia were independently associated with relapse (173).
Several studies have already shown that low CD4+ T lymphocyte counts during the active phase of VL or even the absence of an increase in this subpopulation after treatment can be considered a predictive factor for VL relapse in HIV-infected patients (178–184). In this way, previous studies by our group have demonstrated that the number of VL episodes is inversely correlated with the CD4+ T-cell count and sjTREC level in patients with VL/HIV co-infection (15, 16) and could be a useful immunological biomarker for disease relapse. Interestingly, patients with relapsing VL without a history of HIV also maintained low CD4 T-cell counts post-treatment (32). These studies reinforce the relationship between the degree of immunological reconstitution and different clinical outcomes of the disease.
Bhattacharyya et al. showed that high levels of anti-SLA (soluble Leishmania antigen) immunoglobulin (Ig)G1 at 6 months post-treatment were associated with treatment failure and relapse (185), demonstrating the importance of IgG1 in determining the clinical status of patients with VL, without HIV. These findings were confirmed by subsequent studies that demonstrated the possibility of using IgG1 anti-rK39 antibodies as biomarkers for VL relapse (186, 187). Similarly, Mondal et al. demonstrated the potential of quantifying serum IgG anti-rK39 antibodies to determine VL prognosis (188). Corroborating these data, our group demonstrated that patients with several episodes of VL showed high levels of anti-SLA immunoglobulins, especially IgG3, even 12 months post-treatment and independent of the HIV serological status (15, 32). These studies point to IgG3 as a possible biomarker of relapse, and indicate that a reduction in its serum levels may be related to the clinical remission of VL.
In conclusion, despite the considerable prevalence of VL relapse in patients co-infected with HIV and, more recently, in immunocompetent patients, official protocols for recognizing patients susceptible to relapses are lacking, and their therapeutic management is not well-defined. This scenario, combined with the fact that available treatments are scarce, have significant toxicity, and pose a high cost to the health system, highlight the need to investigate the reactivation-related immunological mechanisms that can help predict the clinical prognosis of these patients (Figure 2). Finally, microbial translocation (sCD14), exhaustion (PD-1/TIGIT)- and senescence (TREC)-associated markers along with immune activation profile (IgG1/IgG3 and CD38/HLA-DR) deserve to be better investigated as underlying determinants of relapse or chronicity of VL in patients with HIV co-infection, in addition to being evaluated using algorithms to validate them as prognostic biomarkers (Figure 1, Table 1). Finally, the cross-sectional studies on VL/HIV conducted to date have provided evidence of the global immunological impairment experienced by these patients. However, prospective multicenter studies are crucial to investigate which immunological parameters can be good predictors of prognosis in terms of VL relapse and/or severity in patients with VL/HIV co-infection.
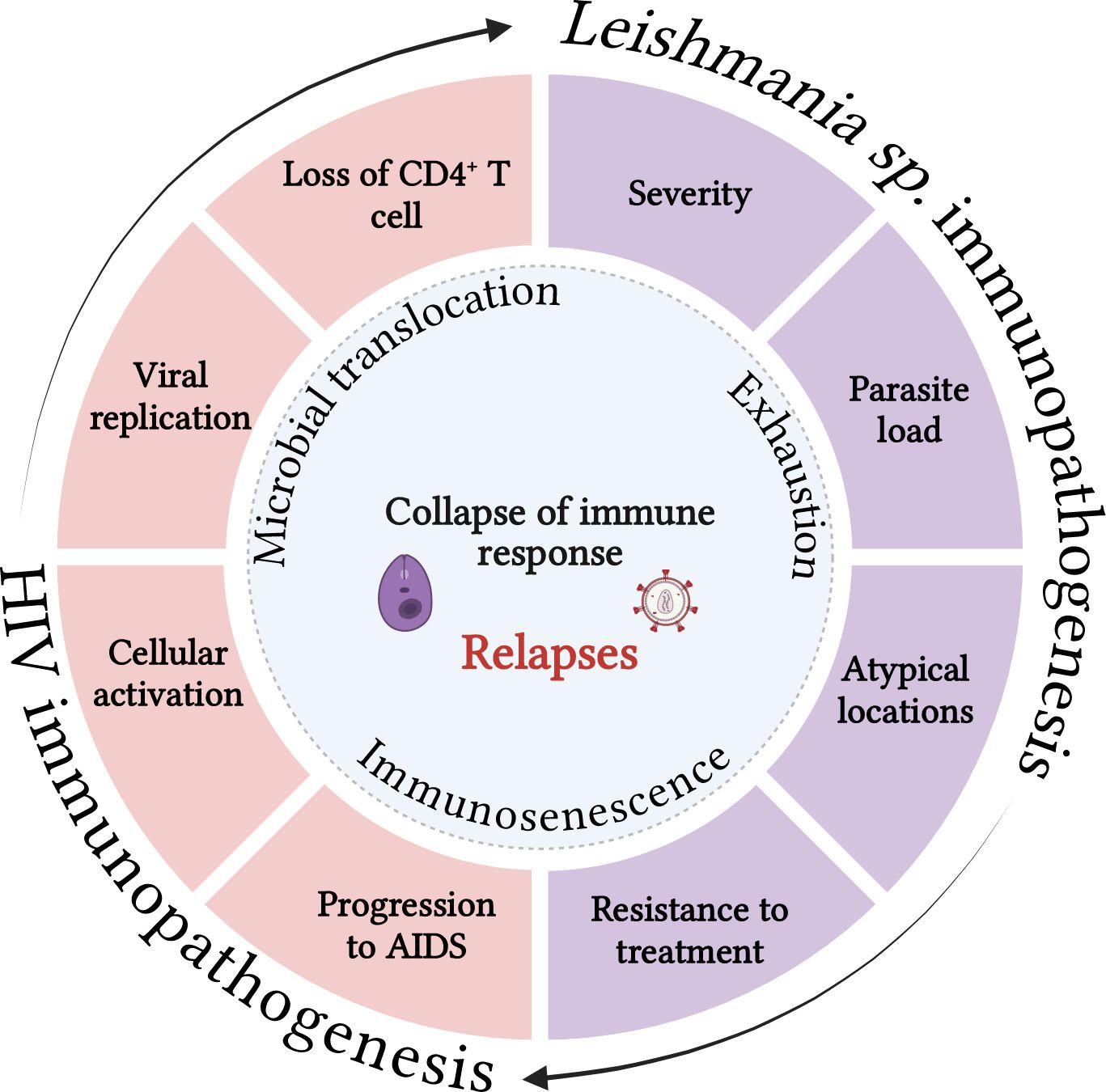
Figure 2. Both infections share immunopathogenic characteristics that can reciprocally impair the immune response to pathogens. Visceral leishmaniasis can contribute to the decrease in CD4 T lymphocytes, worsening immunosuppression, increasing viral replication and the cellular activation degree, which in turn may favor progression to AIDS. On the other hand, HIV infection and its consequences for the immune response can increase the parasite load, the resistance to the treatment, dissemination of VL for atypical sites and favor the severity and relapses of the disease. Microbial translocation, immunosenescence and the exhaustion degree may act as key cofactors of the process that culminates in the collapse of the immune response in co-infected patients. Source: The author. BioRender.com.
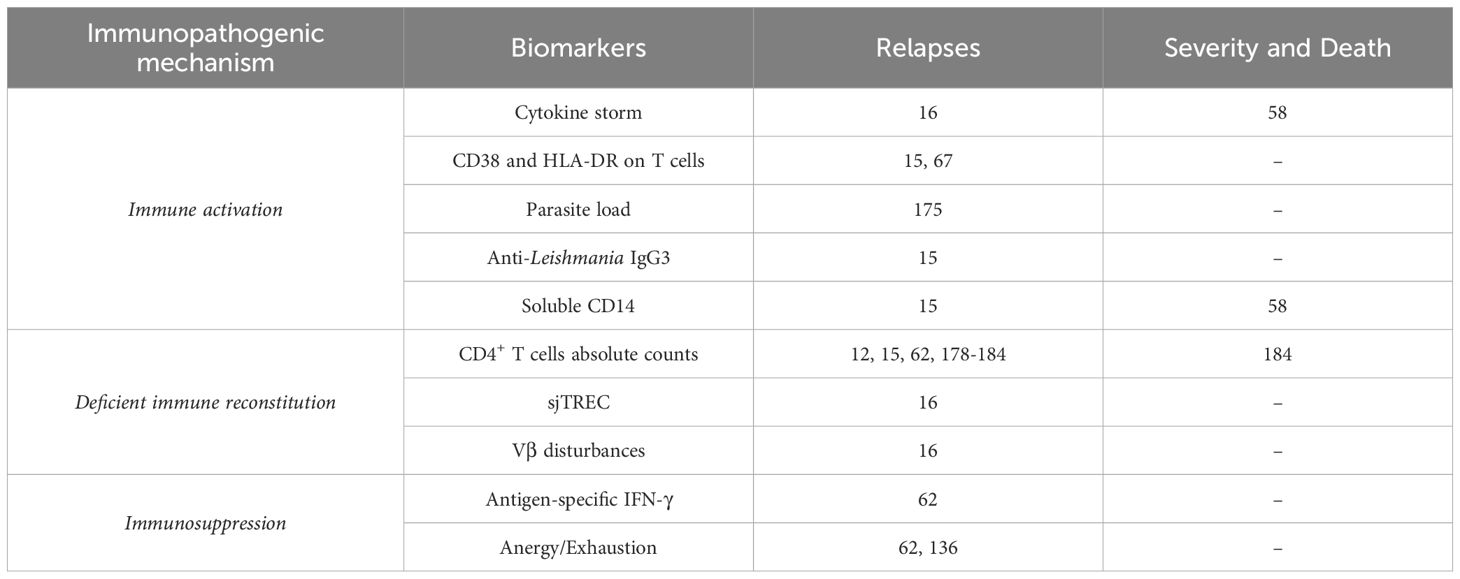
Table 1. Potential biomarkers related to severity, death or relapses of VL in HIV co-infected patients.
Author contributionsMS-F: Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Methodology. GC-C: Conceptualization, Writing – original draft. AMD-C: Conceptualization, Writing – review & editing, Formal analysis, Funding acquisition. JS-O: Conceptualization, Writing – review & editing, Funding acquisition, Supervision, Writing – original draft.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. MLS-F received a scholarship from Coordenação de Vigilância em Saúde e Laboratórios de Referência – Presidência – Fundação Oswaldo Cruz (PRES-009-FIO-22) or FAPERJ (PDR-10 – 200.388/2024 and 200.389/2024); GC-C received a scholarship from Conselho Nacional de Desenvolvimento Cientı́fico e Tecnológico – CNPq/Brasil (PDJ - 150686/2024-2); AMD-C received a funding from FAPERJ (CNE: E-26/201.037/2021) and is a CNPq 1C scholarship holder (305606/2022-0); JRS-O received a funding from IFRJ (Pro-Ciência/2024) and FAPERJ (APQ-1 - E-26/211.693/2021).
AcknowledgmentsWe thank the support team at Hospital Eduardo de Menezes - HEM, and all participants in our studies.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GlossaryVL: visceral leishmaniasis
HIV: human immunodeficiency virus
VL/HIV: visceral leishmaniasis and human immunodeficiency virus co-infection
AIDS: acquired immunodeficiency syndrome
cART: combined antiretroviral therapy
CD: cluster of differentiation
IL: interleukin
IFN: interferon
TNF: tumor necrosis factor
RANTES: normal T-cell expressed and secreted
MIP: macrophage inflammatory protein
CXCL: chemokine ligand
NF: nuclear factor
MDM: monocyte-derived macrophages
PI: protease inhibitor
IRIS: immune reconstitution inflammatory syndrome
PKDL: post-kala-azar dermal leishmaniasis
HLA: Human Leucocyte Antigen
SIV: simian immunodeficiency virus
CCR: chemokine receptor
I-FABP: intestinal fatty-acid binding protein
LPS: lipopolysaccharide
sCD14: soluble receptor CD14
TLR: Toll-like receptor
MALT: mucosa-associated lymphoid tissue
MIF: macrophage migration inhibitory factor
PD-1: programmed cell death protein 1
CTLA-4: cytotoxic T-lymphocyte–associated antigen 4
LAG-3: lymphocyte-activation gene 3
TIM-3: T-cell immunoglobulin and mucin domain-containing protein 3
PD-L1: programmed cell death prote
Comments (0)