Adrenocortical carcinoma (ACC) is a rare and highly invasive cancer emerging from the adrenal cortex, with an estimated incidence rate of about 0.7-2 cases per million individuals globally each year (1–5). The age profile of ACC follows a bimodal pattern with significant peaks in early childhood, around age 5, and reoccurring in the age range of 40s to 50s (1, 6, 7). The survival rate over a 5-year period for ACC varies significantly by stage, ranging from approximately 60-80% for tumors localized, 35-50% for locally advanced cases, and less than 30% for those with distant metastasis (5, 8). For localized tumors treated with surgery and adjuvant therapy, survival rates ranged from 75% to 82% (7, 9). Recurrence after curative surgical treatment remains a significant challenge, with recent studies reporting a recurrence rate of 70% among patients with stage I-III and a median disease-free survival (DFS) duration lasting 11 months following surgery (10). Pediatric patients generally show better outcomes, especially those aged 4 years or younger, with survival rates ranging from 54.2% to 91% (9, 11).
ACC prognosis is influenced by various clinical factors, including patient age, tumor stage, hormone secretion, tumor grade, Ki-67 index, somatic gene mutations, methylation profiles, microRNA and gene expression patterns, and tumor margin status (9). Complete tumor resection through surgery remains the sole curative option for ACC. However, recurrence rates vary significantly depending on the tumor grade, with lower-grade tumors having a recurrence rate of approximately 20-30%, while higher-grade tumors can have recurrence rates exceeding 70%. Due to these high rates of recurrence, the use of adjuvant treatment is critical to improve long-term outcomes and reduce the likelihood of cancer recurrence. Mitotane is the single drug approved by the Food and Drug Administration (FDA), utilized both as adjuvant therapy and in advanced disease stages (12, 13). However, the treatment options for ACC are often limited and insufficient, especially in advanced stages, due to its heterogeneous nature, which leads to varied responses to conventional therapies. First-line treatment (EDP-M), which involves mitotane along with etoposide, doxorubicin, and cisplatin, shows low response rates, with approximately 1.3% of patients attaining a complete response (CR), while 19.2% show a partial response (PR) (5, 14). Given the unsatisfactory outcomes of current therapies, an urgent need exists for novel treatment options to enhance survival rates, reduce recurrence risk, and address drug resistance.
Recent advances in whole-genome molecular techniques combined with large-scale multi-omics studies have enabled high-resolution multi-platform profiling of large cohorts of ACC and have provided novel insights into its molecular pathogenesis (15). Comprehensive multi-omics studies have greatly enhanced our understanding of the molecular mechanisms underlying adrenocortical carcinogenesis. Notably, the TCGA-ACC project has defined three distinct molecular subtypes, COC1, COC2, and COC3. COC1 has the best prognosis, while COC2 has an intermediate prognosis, and COC3 exhibits the worst prognosis. This molecular classification refines our understanding of ACC through multi-omics integration and further identifies subtype-specific therapeutic vulnerabilities (15). Genetic alterations identified so far in ACC span key pathways, among which are Wnt/β-catenin signaling (16, 17), insulin-like growth factor 2/insulin-like growth factor 1 receptor (IGF2/IGF1R) pathway (18–21), cell cycle and p53-mediated apoptosis (16, 22), telomere maintenance (23, 24), cAMP-PKA signaling (25, 26), covering multiple steps involved in transcription and translation. The ACC multi-omics studies have discovered unique molecular subtypes, potential therapeutic targets, and prognostic biomarkers with research covering mRNA, miRNA, DNA methylation, proteomics, and metabolomics (27–30). The influence of the immune microenvironment on drug sensitivity has also been investigated (31). Xenografts and genetically engineered mouse models are utilized to evaluate anticancer therapies, contributing to a more profound understanding of ACC pathogenesis (32, 33). This review will discuss recent findings in genomics, progress in targeted therapies, and ongoing research in immunotherapy, with the aim of specifying the therapeutic landscape and future directions in the personalized treatment, with particular attention to ACC in adults.
2 Genomic and molecular mechanisms2.1 Core genetic pathways in ACC pathogenesisComprehensive genomic studies of ACC have revealed recurrent mutations in critical pathways, including Wnt/β-catenin (ZNRF3, CTNNB1), p53-driven apoptosis and cell cycle regulation (CDKN2A, CDK4, RB1, TP53), chromatin remodeling and telomere stabilization (MEN1, DAXX, TERT, TERF2, ATRX), cAMP-PKA signaling (PRKAR1A), and the IGF2/IGF1R axis, all of which contribute to dysregulated cell proliferation, impaired apoptosis, and an unfavorable prognosis (9).
The IGF2/IGF1R axis is crucial in the progression of ACC, with IGF2 overexpression occurring in nearly 90% of cases (30). This overexpression drives tumor cell proliferation by stimulating IGF1R and subsequent signaling pathways, including the PI3K/AKT/mTOR and RAS/RAF/MEK/ERK pathways, and is often associated with epigenetic changes at the 11p15 locus (30, 34). This dysregulation contributes to tumor growth and resistance to apoptosis, positioning IGF2/IGF1R as an important target for therapy in ACC. The Wnt/β-catenin pathway (8, 9, 15, 30) is implicated in up to 54% of ACC cases and is crucial in the development of ACC, with CTNNB1 mutations causing β-catenin accumulation that drives cell proliferation and survival. ZNRF3 mutations, which disrupt Wnt pathway inhibition, further enhance β-catenin signaling in ACC. TP53 mutations in ACC disrupt the p53 tumor suppressor pathway, leading to uncontrolled cell proliferation, DNA damage accumulation, and increased genomic instability; these mutations are notably common in Li-Fraumeni syndrome and sporadic ACC. Additionally, alterations in cell cycle regulators such as CDKN2A, RB1, CDK4, and CCNE1 drive unchecked proliferation, with RB1 or CDKN2A loss removing growth checkpoints and CDK4 and CCNE1 amplification accelerating the cell cycle, often correlating with more aggressive ACC and poorer prognosis (9, 15, 30, 35).
2.2 Growth factor and cytokine-mediated signaling pathwaysMultiple growth factors and cytokines, including transforming growth factor-α (TGF-α) (35), transforming growth factor-beta 1 (TGF-β1) (35), vascular endothelial growth factor (VEGF) (35, 36), fibroblast growth factor (FGF-2) (9, 35), and several interleukins (35), play essential roles in ACC progression. These molecules bind to tyrosine kinase-coupled receptors (TKRs) on cell surfaces, activating signaling cascades that regulate critical cellular processes. Specifically, TGF-α and TGF-β1 contribute to cell proliferation and immune modulation, aiding tumor growth and evasion of immune responses. VEGF primarily supports angiogenesis, which supplies nutrients to the tumor and is crucial for sustained growth and potential metastasis. FGF-2, along with VEGF, enhances cell survival and proliferation, further promoting tumor aggressiveness. The combined effect of these pathways in ACC leads to increased cellular resistance to apoptosis, heightened angiogenic potential, and an enhanced capacity for metastatic spread, which collectively worsen the prognosis.
2.3 DNA damage repair mechanismsAlterations in DNA damage repair (DDR) pathways are frequently detected in ACC, affecting various pathways crucial for maintaining genomic stability. Notable mutations have been identified in genes responsible for damage sensing (such as ATR, ATM, CHEK2), mismatch repair (including MLH1, MSH2, MSH6), and homologous recombination (such as BRCA1, BRCA2, RAD51) (8, 9). These mutations disrupt essential DDR pathways, leading to DNA damage accumulation, genomic instability, and promoting tumor progression. Additionally, germline mutations in mismatch repair (MMR) genes link certain ACC cases to Lynch syndrome, a hereditary cancer syndrome. Studies, including those by TCGA, have identified additional driver mutations in tumor suppressor genes like NF1 and MLL4, further compromising DDR functions.
2.4 Chromatin remodeling and epigenetic modificationsChromatin remodeling and epigenetic changes-such as DNA methylation at CpG islands, histone modification, and telomere maintenance-play key roles in tumor progression and prognosis (9, 15). Abnormal DNA methylation, including high levels of CpG island methylator phenotype (CIMP), results in the inactivation of tumor suppressor genes and is associated with poor outcomes. TERT promoter mutations increase telomerase expression, bypassing cellular senescence, while mutations in genes like EZH2, ATRX, and DAXX alter chromatin structure and transcription, promoting tumor growth and progression (9). Blocking chromatin remodeling enzymes, including histone-lysine N-methyltransferases KMT2A–KMT2D, menin, as well as histone-lysine N-methyltransferase SETD2, may offer a promising approach for targeted therapy.
2.5 Non-coding RNA and post-transcriptional regulationIn ACC, non-coding RNAs, including microRNAs (such as miR-483-5p) and long non-coding RNAs (like H19 and UCA1), participate in the regulation of gene expression post-transcription, impacting tumor aggressiveness, proliferation, and survival (9, 37). Dysregulated microRNAs often target tumor suppressor genes or oncogenes, promoting cancer progression, while lncRNAs influence pathways that support tumor growth and resistance to apoptosis.
3 Targeted therapy for ACCMitotane, a DDT derivative with adrenal toxicity, inhibits Sterol O-Acyltransferase 1 (SOAT1) in ACC cells, disrupting cholesterol balance and enhancing oxidative stress sensitivity to suppress tumor growth. Additionally, it induces cellular necrosis, inhibits steroidogenesis by targeting CYP11A1 and CYP11B1, and strongly induces CYP3A4, potentially reducing the efficacy of concurrent medications (38). Clinical trials, including the ADIUVO trial (39–41), are investigating the role of mitotane in post-surgical patients. Although cytotoxic chemotherapy combined with mitotane has shown efficacy in advanced cases, its objective response rate (ORR) in advanced ACC is limited to around 24% (5, 42). Additionally, these treatment regimens often lead to significant toxicities, particularly affecting the gastrointestinal and neurological systems, with symptoms such as nausea, vomiting, and headache, which may restrict their long-term use. Given these limitations, the investigation of molecular targeted therapies offers a promising avenue for enhancing personalized treatment strategies for ACC.
3.1 Inhibitors of the IGF-1R and mTOR pathwaysIGF1R inhibitor NVP-AEW541 showed inhibition of tumor cell growth in NCI-H295R and RL251 ACC models, with an enhanced effect when used in combination with mitotane (43). Everolimus (RAD-001), an mTOR inhibitor, has been shown to reduce tumor cell growth both in vitro and in vivo in xenograft models using NCI-H295R cells (44). Early clinical attempt of IGF1R inhibitors, including linsitinib (OSI-906) and cixutumumab (IMC-A12), have demonstrated some preliminary activity against ACC, including partial responses in certain cases (18, 45). A phase 1 study (46) demonstrated the combination therapy of cixutumumab and temsirolimus (an mTOR inhibitor) resulted in extended disease stability, with 42% patients maintaining stability for over 6 months; however, limitations such as limited overall efficacy, significant toxicities (e.g., grade 4 hyperglycemia and multiorgan failure), and potential patient selection bias have hindered the continued development of these therapies for advanced ACC.
3.2 Inhibitors targeting Wnt/β-catenin signalingPreclinical studies targeting Wnt/β-catenin signaling, involving small-molecule inhibitor PKF115-584 to block the TCF/β-catenin complex and doxycycline-triggered β-catenin suppression via shRNA, demonstrated reduced tumor cell proliferation and triggered apoptosis in cell culture studies, along with full inhibition of tumor growth in vivo. Nonetheless, the clinical efficacy of Wnt pathway inhibitors has yet to be fully established, and additional research is required to evaluate their potential benefits and limitations in treating ACC (9).
3.3 Inhibitors of tyrosine kinase receptorsResearch into tyrosine kinase inhibitors (TKIs) for ACC has shown varied outcomes across different agents. Cabozantinib, which inhibits multiple kinases including VEGF, AXL, c-MET, and RET, demonstrated promising efficacy in a phase 2 trial with an overall survival of 16 weeks in advanced ACC patients, suggesting it may be a viable option following failure of other treatments (47). In a phase 2 trial involving advanced ACC patients, cabozantinib achieved a progression-free survival (PFS) rate of 72.2% at 4 months, with a median PFS of 6 months. Adverse events of grade 3 or higher were reported in 61% of patients and were considered manageable (48). Studies on Sunitinib, which targets VEGFR and PDGFR, indicated limited efficacy, with a PFS of less than 3 months in patients with refractory ACC, partially due to drug interactions with mitotane, which decreases TKI blood levels (49). Sorafenib, another TKI with activity against VEGFR and RAF kinase, was also tested in ACC but failed to produce sustained tumor control, especially when combined with chemotherapy, leading to early trial termination (50, 51). Other agents, such as Dovitinib (an FGFR inhibitor) and Axitinib (a VEGFR inhibitor), showed minimal impact in terms of objective response rates, with Dovitinib achieving only stable disease in less than a quarter of patients for a duration exceeding 6 months (36). Additionally, targeting both EGFR and IGF1R in ACC through combined TKI therapy (Erlotinib and IGF1R inhibitor NVP-AEW541) has been explored preclinically, showing enhanced tumor inhibition compared to single-agent approaches, highlighting the potential for synergistic effects in dual-targeted strategies (52).
3.4 PPARγ agonistsAgonists of peroxisome proliferator-activated receptor gamma (PPARγ), including pioglitazone and rosiglitazone, have demonstrated significant anti-tumor effects in ACC through multiple mechanisms in preclinical studies. These agents inhibit tumor cell proliferation and invasiveness, promote apoptosis, and reduce angiogenesis by downregulating pro-survival markers like VEGF and Bcl-2, additionally, by suppressing critical signaling pathways such as PI3K/AKT and extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK2) (9, 53–55). In xenograft models, rosiglitazone treatment resulted in a marked reduction in tumor volume, decreased microvessel density, and increased expression of C-X-C motif chemokine ligand 12 (CXCL12), a signaling protein associated with lower malignancy and better survival (56).
3.5 Inhibitors targeting cell cycle and DNA damage repairNutlin-3a (RG7112), an MDM2 inhibitor, has shown efficacy in reducing tumor growth, inducing apoptosis, and inhibiting cellular proliferation and hormone production in ACC models, especially those with CTNNB1 mutations (57). Preclinical studies indicate that targeting polo-like kinase 1 (PLK-1) with selective inhibitors like BI-2536, alone or combined with MDM2 inhibition, effectively reduces cell viability, restores p53 function, and induces apoptosis in adrenocortical carcinoma (ACC) cells, suggesting a promising therapeutic approach for ACC (58, 59). CDK4/6 inhibitors, such as palbociclib and ribociclib (9, 60), have shown efficacy in vitro by reducing cell viability and inducing senescence or apoptosis in ACC cell lines. Additionally, poly (ADP-Ribose) Polymerase (PARP) inhibitors, such as rucaparib, olaparib, talazoparib and niraparib, have been investigated due to their potential synthetic lethality in DDR-deficient tumors, although their efficacy in ACC specifically requires further investigation. In summary, these studies highlight CDK4/6 inhibitors and DDR-targeting agents as potential therapies. Further clinical trials are required to validate their efficacy and safety.
3.6 Epigenetic modifiers and non-coding RNA therapiesRecent studies on ACC have focused on various epigenetic therapies, including histone deacetylase (HDAC) inhibitors (vorinostat) and DNA methylation inhibitors (decitabine), both of which have shown potential antitumor activity in preclinical models. Vorinostat demonstrated enhanced efficacy when combined with standard chemotherapy, while decitabine exhibited tumor-suppressive effects by reactivating silenced genes and reducing ACC cell proliferation, cortisol secretion, and invasiveness (61–64). The study emphasizes several miRNAs, including miR-483-5p, miR-139-5p, miR-100, miR-34a, miR-184, and miR-181b. Furthermore, long non-coding RNAs such as PRINS, RAD50, and HAND2 are also highlighted for their involvement in tumor progression and association with poor prognosis, suggesting their potential as therapeutic candidates in ACC (9).
3.7 Selective estrogen receptor modulatorsResearch into estrogen and progesterone pathway inhibition in ACC shows promising preclinical findings (9). Hydroxytamoxifen is an active metabolite produced from tamoxifen, which has been shown to increase FASL (Fas ligand, a pro-apoptotic factor) expression, leading to reduce in vitro cellular growth and decreased tumor progression in xenograft models. G-1, which is a nonsteroidal G-protein coupled estrogen receptor (GPER) agonist, demonstrated proliferation inhibition in ACC cells through stimulation of the ERK1/2 signaling pathway in both laboratory and animal models. The inverse agonist XCT790, which targets estrogen-related receptor alpha (ERRα), was shown to impair mitochondrial activity, leading to a decrease in ACC cell proliferation and promoting cell death both in vitro and in animal models.
3.8 Steroidogenesis-targeted therapiesRecent research has identified steroidogenesis inhibitors as promising therapies for ACC, aiming to block key enzymes and pathways involved in hormone production. The acyl-CoA cholesterol acyltransferase 1 (ACAT1) inhibitor, ATR-101, has demonstrated efficacy in preclinical studies by inducing apoptosis in ACC cells through mechanisms such as mitochondrial hyperpolarization, oxygen species (ROS) generation, and activation of endoplasmic reticulum (ER) stress (65, 66). Other compounds, including AC-45594 (an alkyloxyphenol) and OOP (an isoquinolinone), suppress ACC cell proliferation by targeting the transcription factor steroidogenic factor 1 (SF-1), which plays a crucial role in steroid synthesis and is often overexpressed in ACC (67).
4 Immunotherapy approaches in ACCInterest in immunotherapy research has grown because of the limited efficacy that traditional treatments for ACC have shown. Most recently, attention has fallen on immune checkpoint inhibitors (ICIs), IL-13Rα2-targeting, and other forms of precision therapy aimed at a more tailored approach in the treatment of ACC. Specific features in ACC and its immune evasion mechanisms do continue to complicate successful immunotherapeutic approaches in this disease.
Immune checkpoint inhibitors, especially those targeting cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), proved effective in treating various solid tumors, such as melanoma, renal cell carcinoma and non-small cell lung cancer. A number of ACC trials with PD-1 inhibitors have been completed or are in progress, and the majority of those so far utilized pembrolizumab and avelumab, but despite inducing tumor-suppressive effects in some patients, the overall efficacy was limited. Results from single-agent studies, for instance, were quite modest. A phase 2 study revealed that pembrolizumab led to a 23% response rate and a 52% rate of disease control in individuals with advanced adrenal cortex carcinoma, accompanied by a median survival duration of 24.9 months and tolerable safety in microsatellite-stable patients (68). Ipilimumab and nivolumab, when used together, proved effective in patients with refractory metastatic ACC, achieving a 19% immune response rate and 33% clinical benefit rate, with PFS up to 57 months (69). Thus, some patients with ACC do seem to benefit from ICIs, but these drugs do not improve the survival of most appreciably. A recent study revealed that the combination of camrelizumab and apatinib exhibited substantial antitumor effects in previously treated advanced ACC patients, achieving an objective response rate of approximately 50% and a median PFS duration of 12.6 months, with manageable toxicity (70). Exploratory analysis indicated changes in the immune microenvironment, including increased CD8+ and CXCR3+ T cells, reduced immunosuppressive CD4+ T cells, and higher clonal overlap between tumor-infiltrating and circulating T cells, suggesting that these immune alterations may be associated with a favorable treatment response. While ICIs show potential benefits for some ACC patients, further research is needed to address sample size, limited effectiveness, drug toxicity, patient selection in trials, and combination therapy strategies.
Thus, IL-13Rα2 has become an attractive target. Very highly overexpressed in the cells of ACC but minimally in normal adrenal tissue, IL-13Rα2 is an attractive target for immunotherapy. IL-13-PE, a chimeric protein comprising IL-13, specifically targets ACC cells that express IL-13Rα2. In the Phase 1 trial of IL-13-PE, three out of six IL-13Rα2-positive ACC patients developed disease stability lasting for 2 to 5.5 months, suggesting that IL-13Rα2-targeting immunotherapy may have antitumor efficacy and could be a novel treatment for ACC in the future (71). Research is also being conducted on chimeric antigen receptor T-cells (CAR-T) that target IL-13Rα2 (72). This method involves engineering T cells from a patient to recognize and attack ACC cells expressing IL-13Rα2. Preclinical results have demonstrated promising antitumor efficacy, indicating that IL-13Rα2-targeting therapies may be further developed to play a more important role in ACC immunotherapy.
5 Conclusion and future directionsAdvances in molecular and genetic research have considerably enlightened the rare and aggressive nature of adrenocortical carcinoma over recent years. Molecular-targeted therapies have also promised a new ray of hope through specific inhibitions crucial for the growth and differentiation of ACC cells. A overview of key molecular drivers and drug targets in ACC is shown in Figure 1. However, the low efficacy detected in clinical trials so far underlines that big challenges are yet to be overcome in the identification of reliable molecular drivers and effective therapeutic outcomes. Current limitations include bypass pathway activations, lack of precise molecular targets for patient stratification, and insufficient response to single-agent therapies. These findings emphasize that more detailed knowledge about the molecular mechanism of ACC is necessary to guide individualized treatment options.
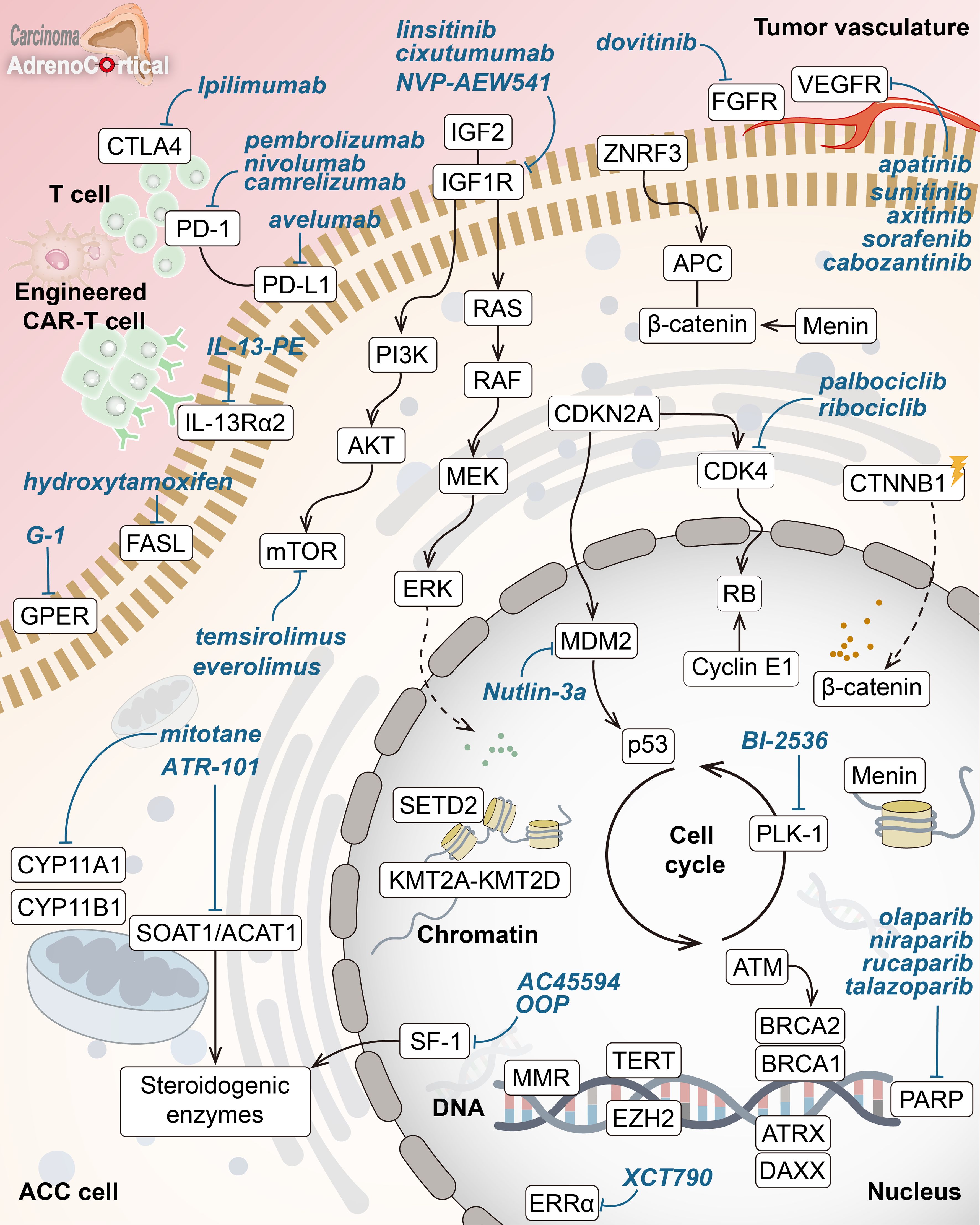
Figure 1. Key molecular drivers and drug targets in adrenocortical carcinoma. Overview of core pathways in adrenocortical carcinoma (ACC) pathogenesis with potential targeted therapies (in blue). Key hallmarks include abnormal Wnt/β-catenin signaling, disrupted cell cycle (p53-RB, CDK4), chromatin remodeling inhibition (menin, KMT2A-KMT2D, SETD2), DNA repair defects (MMR enzymes, ATM, BRCA1/2, PARP), altered telomere maintenance (ATRX, DAXX, TERT), and abnormal steroid metabolism. Highlighted treatments target IGF1R, mTOR, VEGFR, β-catenin, SOAT1, and ERRα, along with immune checkpoint inhibitors and other pathways.
Future treatment strategies for ACC are anticipated to combine molecular diagnostics, targeted therapies, and immunotherapy. The constant identification of actionable biomarkers and genetic signatures provides hope for diagnostics, as these facilitate the prompt identification and classification of patients who may respond to targeted treatments. Biomarker-driven clinical trials require emphasis in ongoing research to bring personalized approaches to ACC management. Lastly, although immunotherapy has challenges like low mutational burden and immune evasion mechanisms, the approach seems promising when combined with other therapies. Preliminary data indicate that ICIs may provide clinical benefit when combined with VEGF inhibitors or other targeted therapies. It is also multidisciplinary and a team approach. Centers of excellence and international collaboration in research can accelerate the transform of basic research to clinical applications. The use of multi-omics technologies and next-generation sequencing may, in the future, provide a better insight into ACC-specific biological features and enable the drug repositioning active in other cancers for ACC treatment. In addition, some novel therapeutic approaches or concepts, such as some artificial intelligence-guided, voluntary exercise-combined or nanomaterials-based therapies, have provided potential directions for ACC treatment in future, yet rigorous preclinical validation and clinical trials are still warranted to confirm their clinical perspective and therapeutic efficacy (73–77). Further research should be targeted toward resistance mechanisms elucidation, optimization of combination regimens, and early detection and therapeutic strategies that could potentially enhance survival rates and overall well-being for patients with ACC.
Author contributionsJS: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. JH: Writing – original draft. WZ: Formal analysis, Writing – review & editing. TZ: Writing – review & editing. RS: Visualization, Writing – original draft. XW: Investigation, Writing – review & editing. ML: Supervision, Validation, Writing – review & editing. XJ: Supervision, Writing – review & editing. XZ: Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82203850 to JS; No. 82274155 to XW) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Jiangsu Graduate Innovation Project (SJCX23-0785).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GlossaryACC: adrenocortical carcinoma
ATR: ataxia telangiectasia and Rad3 related
ATM: ataxia telangiectasia mutated
BRCA1: breast cancer 1
BRCA2: breast cancer 2
CHEK2: checkpoint kinase 2
CTLA-4: cytotoxic T-lymphocyte associated protein 4
CYP11A1: cytochrome P450 family 11 subfamily A member 1
CYP11B1: cytochrome P450 family 11 subfamily B member 1
CYP3A4: cytochrome P450 family 3 subfamily A member 4
DAXX: death domain associated protein
DDR: DNA damage repair
EDP-M: etoposide, doxorubicin, and cisplatin with mitotane
EZH2: enhancer of zeste homolog 2
FDA: Food and Drug Administration
FGF-2: fibroblast growth factor 2
ICIs: immune checkpoint inhibitors
IGF1R: insulin-like growth factor 1 receptor
IGF2: insulin-like growth factor 2
IL-13Rα2: interleukin 13 receptor alpha 2
KMT2A–KMT2D: Lysine (K) Methyltransferase 2A–2D
lncRNA: long non-coding RNA
miRNA: microRNA
MLH1: MutL homolog 1
MLL4: mixed lineage leukemia 4
MSH2: MutS homolog 2
MSH6: MutS homolog 6
mTOR: mechanistic target of rapamycin
NF1: neurofibromin 1
PD-1: programmed cell death protein 1
PPARγ: peroxisome proliferator-activated receptor gamma
RAD51: RAD51 recombinase
SETD2: SET Domain Containing 2
TCGA: The Cancer Genome Atlas
TERT: telomerase reverse transcriptase
TGF-α: transforming growth factor-alpha
TGF-β1: transforming growth factor-beta 1
TKI: tyrosine kinase inhibitor
VEGF: vascular endothelial growth factor
References1. Shariq OA, McKenzie TJ. Adrenocortical carcinoma: current state of the art, ongoing controversies, and future directions in diagnosis and treatment. Ther Adv Chronic Disease. (2021) 12:20406223211033103. doi: 10.1177/20406223211033103
PubMed Abstract | Crossref Full Text | Google Scholar
2. Sharma E, Dahal S, Sharma P, Bhandari A, Gupta V, Amgai B, et al. The characteristics and trends in adrenocortical carcinoma: A united states population based study. J Clin Med Res. (2018) 10(8):636–40. doi: 10.14740/jocmr3503w
PubMed Abstract | Crossref Full Text | Google Scholar
3. Kerkhofs TM, Verhoeven RH, van der Zwan JM, Dieleman J, Kerstens MN, Links TP, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the netherlands since 1993. Eur J Cancer. (2013) 49(11):2579–86. doi: 10.1016/j.ejca.2013.02.034
PubMed Abstract | Crossref Full Text | Google Scholar
4. Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. (2014) 99(2):455–61. doi: 10.1210/jc.2013-2856
PubMed Abstract | Crossref Full Text | Google Scholar
5. Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. (2012) 366(23):2189–97. doi: 10.1056/NEJMoa1200966
PubMed Abstract | Crossref Full Text | Google Scholar
7. Tella SH, Kommalapati A, Yaturu S, Kebebew E. Predictors of survival in adrenocortical carcinoma: An analysis from the national cancer database. J Clin Endocrinol Metab. (2018) 103(9):3566–73. doi: 10.1210/jc.2018-00918
PubMed Abstract | Crossref Full Text | Google Scholar
9. Ghosh C, Hu J, Kebebew E. Advances in translational research of the rare cancer type adrenocortical carcinoma. Nat Rev Cancer. (2023) 23(12):805–24. doi: 10.1038/s41568-023-00623-0
PubMed Abstract | Crossref Full Text | Google Scholar
10. Glenn JA, Else T, Hughes DT, Cohen MS, Jolly S, Giordano TJ, et al. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery. (2019) 165(1):186–95. doi: 10.1016/j.surg.2018.04.068
PubMed Abstract | Crossref Full Text | Google Scholar
11. McAteer JP, Huaco JA, Gow KW. Predictors of survival in pediatric adrenocortical carcinoma: a surveillance, epidemiology, and end results (SEER) program study. J Pediatr Surg. (2013) 48(5):1025–31. doi: 10.1016/j.jpedsurg.2013.02.017
PubMed Abstract | Crossref Full Text | Google Scholar
12. Puglisi S, Calabrese A, Basile V, Pia A, Reimondo G, Perotti P, et al. New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. (2020) 34(3):101415. doi: 10.1016/j.beem.2020.101415
PubMed Abstract | Crossref Full Text | Google Scholar
13. Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. New Engl J Med. (2007) 356(23):2372–80. doi: 10.1056/NEJMoa063360
PubMed Abstract | Crossref Full Text | Google Scholar
14. Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, et al. European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the european network for the study of adrenal tumors. Eur J Endocrinol. (2018) 179(4):G1–G46. doi: 10.1530/EJE-18-0608
PubMed Abstract | Crossref Full Text | Google Scholar
15. Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. (2016) 29(5):723–36. doi: 10.1016/j.ccell.2016.04.002
PubMed Abstract | Crossref Full Text | Google Scholar
16. Borges KS, Pignatti E, Leng S, Kariyawasam D, Ruiz-Babot G, Ramalho FS, et al. Wnt/β-catenin activation cooperates with loss of p53 to cause adrenocortical carcinoma in mice. Oncogene. (2020) 39(30):5282–91. doi: 10.1038/s41388-020-1358-5
PubMed Abstract | Crossref Full Text | Google Scholar
18. Catalano R, Giardino E, Treppiedi D, Mangili F, Morelli V, Elli FM, et al. The cytoskeleton actin binding protein filamin a impairs both IGF2 mitogenic effects and the efficacy of IGF1R inhibitors in adrenocortical cancer cells. Cancer Lett. (2021) 497:77–88. doi: 10.1016/j.canlet.2020.10.022
PubMed Abstract | Crossref Full Text | Google Scholar
19. Pereira SS, Monteiro MP, Costa MM, Moreira Â, Alves MG, Oliveira PF, et al. IGF2 role in adrenocortical carcinoma biology. Endocrine. (2019) 66(2):326–37. doi: 10.1007/s12020-019-02033-5
PubMed Abstract | Crossref Full Text | Google Scholar
20. Tamburello M, Altieri B, Sbiera I, Sigala S, Berruti A, Fassnacht M, et al. FGF/FGFR signaling in adrenocortical development and tumorigenesis: novel potential therapeutic targets in adrenocortical carcinoma. Endocrine. (2022) 77(3):411–8. doi: 10.1007/s12020-022-03074-z
PubMed Abstract | Crossref Full Text | Google Scholar
21. Sbiera I, Kircher S, Altieri B, Lenz K, Hantel C, Fassnacht M, et al. Role of FGF receptors and their pathways in adrenocortical tumors and possible therapeutic implications. Front Endocrinol (Lausanne). (2021) 12:795116. doi: 10.3389/fendo.2021.795116
PubMed Abstract | Crossref Full Text | Google Scholar
22. Huang Y, Li D, Wang L, Su X, Tang X. CENPF/CDK1 signaling pathway enhances the progression of adrenocortical carcinoma by regulating the G2/M-phase cell cycle. J Transl Med. (2022) 20(1):78. doi: 10.1186/s12967-022-03277-y
PubMed Abstract | Crossref Full Text | Google Scholar
23. Sun H, Chen G, Guo B, Lv S, Yuan G. Potential clinical treatment prospects behind the molecular mechanism of alternative lengthening of telomeres (ALT). J Cancer. (2023) 14(3):417. doi: 10.7150/jca.80097
PubMed Abstract | Crossref Full Text | Google Scholar
25. Huang Y, Zhang W, Cui N, Xiao Z, Zhao W, et al. Fluorene-9-bisphenol regulates steroidogenic hormone synthesis in H295R cells through the AC/cAMP/PKA signaling pathway. Ecotoxicology Environ Safety. (2022) 243:113982. doi: 10.1016/j.ecoenv.2022.113982
PubMed Abstract | Crossref Full Text | Google Scholar
26. Rizk-Rabin M, Chaoui-Ibadioune S, Vaczlavik A, Ribes C, Polak M, Ragazzon B, et al. Link between steroidogenesis, the cell cycle, and PKA in adrenocortical tumor cells. Mol Cell Endocrinology. (2020) 500:110636. doi: 10.1016/j.mce.2019.110636
PubMed Abstract | Crossref Full Text | Google Scholar
27. Sun-Zhang A, Juhlin CC, Carling T, Scholl U, Schott M, Larsson C, et al. Comprehensive genomic analysis of adrenocortical carcinoma reveals genetic profiles associated with patient survival. ESMO Open. (2024) 9(7):103617. doi: 10.1016/j.esmoop.2024.103617
PubMed Abstract | Crossref Full Text | Google Scholar
28. Bi G, Liang J, Zheng Y, Li R, Zhao M, Huang Y, et al. Multi-omics characterization and validation of invasiveness-related molecular features across multiple cancer types. J Transl Med. (2021) 19(1):124. doi: 10.1186/s12967-021-02773-x
PubMed Abstract | Crossref Full Text | Google Scholar
29. Martin-Hernandez R, Espeso-Gil S, Domingo C, Latorre P, Hervas S, Hernandez Mora JR, et al. Machine learning combining multi-omics data and network algorithms identifies adrenocortical carcinoma prognostic biomarkers. Front Mol Biosci. (2023) 10:1258902. doi: 10.3389/fmolb.2023.1258902
PubMed Abstract | Crossref Full Text | Google Scholar
30. Lerario AM, Mohan DR, Hammer GD. Update on biology and genomics of adrenocortical carcinomas: Rationale for emerging therapies. Endocr Rev. (2022) 43(6):1051–73. doi: 10.1210/endrev/bnac012
PubMed Abstract | Crossref Full Text | Google Scholar
31. Lai G, Liu H, Deng J, Li K, Zhang C, Zhong X, et al. The characteristics of tumor microenvironment predict survival and response to immunotherapy in adrenocortical carcinomas. Cells. (2023) 12(5):755. doi: 10.3390/cells12050755
PubMed Abstract | Crossref Full Text | Google Scholar
32. Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. (2014) 46(6):607–12. doi: 10.1038/ng.2953
PubMed Abstract | Crossref Full Text | Google Scholar
33. Wilmouth JJ, Olabe J, Garcia-Garcia D, Lucas C, Guiton R, Roucher-Boulez F, et al. Sexually dimorphic activation of innate antitumor immunity prevents adrenocortical carcinoma development. Sci Advances. (2022) 8(41):eadd0422. doi: 10.1126/sciadv.add0422
PubMed Abstract | Crossref Full Text | Google Scholar
34. Angelousi A, Kyriakopoulos G, Nasiri-Ansari N, Karageorgou M, Kassi E. The role of epithelial growth factors and insulin growth factors in the adrenal neoplasms. Ann Trans Med. (2018) 6(12):253. doi: 10.21037/atm.2018.05.52
PubMed Abstract | Crossref Full Text | Google Scholar
35. Chukkalore D, MacDougall K, Master V, Bilen MA, Nazha B. Adrenocortical carcinomas: Molecular pathogenesis, treatment options, and emerging immunotherapy and targeted therapy approaches. Oncologist. (2024) 29(9):738–46. doi: 10.1093/oncolo/oyae029
PubMed Abstract | Crossref Full Text | Google Scholar
36. O'Sullivan C, Edgerly M, Velarde M, Wilkerson J, Venkatesan AM, Pittaluga S, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. (2014) 99(4):1291–7. doi: 10.1210/jc.2013-2298
Comments (0)