COVID-19 (Coronavirus disease-19) is a multisystem disorder because the virus attaches to the ACE-2 receptors that are found throughout the body and causes direct damage to organs1. The severity of the damage can range from a mild upper respiratory tract infection to disseminated intravascular coagulation that affects multiple organs. Critical complications, such as respiratory failure, myocarditis, and a hypercoagulable state, are common in severe COVID-19 illness. These features may be influenced by endotheliopathy, which is marked by altered endothelial function and increased vascular permeability1,2. COVID-19 and other critical diseases share a common underlying inflammatory process characterised by a cytokine storm. This refers to a significant activation of inflammation in response to an infection, often leading to organ damage and multi-organ failure due to vasculitis, as commonly observed in COVID-19 patients3,4. When the SARS CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) virus infects the lungs, it causes cytokine-driven vascular leaks in the lung’s alveolar-endothelial interface. There are limited reports on endothelial factors as biomarkers for COVID-19 severity5,6.
These biomarkers are closely linked to understanding viral pathogenesis mechanisms, cellular damage, and organ damage. The identification of effective biomarkers would aid clinical management and the prevention of serious complications. Some markers, such as VEGF (Vascular endothelial growth factor), VCAM (Vascular cell adhesion molecule), and ICAM-1(Intercellular Adhesion Molecule-1), are associated with endotheliopathy, while others, like Galectin-37, Angiopoietin-28, and Angiotensin-2, are linked to injuries in different organs, such as the heart, kidney, and lungs. In COVID-19 infections, commonly tested markers such as LDH (Lactate dehydrogenase), CRP (C-Reactive Protein), cytokines, d-dimer, and ferritin are not specific and cannot accurately identify the severity of the infection9,10. To aid clinicians in managing patients during the ongoing waves of the mutating SARS-CoV-2 virus, a panel of specific endothelial biomarkers would be more beneficial in categorising the level of multisystem involvement during COVID-19 infection11. The evaluation of these endothelial markers and an understanding of their dynamics can also be helpful in the early diagnosis and/or prognosis of thrombosis, sepsis, acute respiratory distress syndrome (ARDS)12, and poor clinical outcomes associated not only with COVID-19 but also with other infections that cause multisystem involvement13.
Materials & MethodsThis study was undertaken by the Institute of Microbiology, Madras Medical College, Chennai, Tamil Nadu, after obtaining ethical clearance from the Institutes Ethics Committee.
Study siteThe study was conducted in the Institute of Microbiology in association with Institute of Internal Medicine of the Rajiv Gandhi Government General Hospital (RGGGH), Chennai and patients admitted to RGGGH.
Study designAll the biomarker analysis was performed with serum samples from age-standardised healthy controls and COVID-19 patients representing both ILI (Influenza like Illness)/home isolation and/or SARI (Severe Acute respiratory infections)/hospital admitted patients. It was proposed to employ sample size as follows to get statistically significant data on the level of biomarkers across varied patients against healthy control: healthy control (n=10), ILI/moderate (no complications) COVID-19 patients (n=20), and SARI (n=20; including all SARI COVID-19 manifestations with complications such as cardiac, kidney and pulmonary injury observed in critically ill patients with or without comorbidities).
This study was designed as a pilot initiative with plans to take up in-depth studies in the future. The study was done over six months, from September 2022 to February 2023. Circulatory biomarkers, prominently indicated in COVID-19 compared to ILI cases and healthy controls, were chosen. The markers chosen for this study were vascular endothelial growth factor (VEGF), interleukin 6 (IL6), Angiopoietin-2, ICAM, VCAM, Thrombomodulin, Galectin-3, and TNFR1. The markers that are known to play major roles in COVID-19 pathogenesis were evaluated for the generation profiles to stratify ILI and SARI COVID-19 patients using serum samples against healthy controls.
Study populationAmong the 20 admitted patients,11 of them were admitted to RGGGH, Chennai, and nine were antenatal symptomatic patients admitted to IOG (Institute of Obstetrics and Gynaecology), RGGGH, Chennai. The other 20 patients were COVID-19 positive with features of ILI who were under home isolation after diagnosis and with continuous clinical monitoring by public health officials for complications. The 10 controls were healthy asymptomatic individuals from whom samples were collected. The healthy controls were age and gender-matched with the aforementioned cases and did not have any comorbid conditions or infections during the past two months. Controls were young healthy individuals of both genders from community.
Inclusion/exclusion criteriaAdults, (a) above 18 yr of age with a positive RT PCR test for COVID-19; and (b) laboratory-confirmed COVID-19-positive test among individuals presenting with ILI features under home isolation and patients with moderate to severe ARI manifestations requiring admission were included in this study. COVID-19-positive individuals with secondary bacterial sepsis, on immunosuppression, and individuals with malignant conditions were excluded from the study.
Case definitionSARI cases are defined by the World Health Organization (WHO) as those who have been hospitalised within 10 days after the onset of their illness and have a measurable or reported fever and cough. Patients with ARI (Acute Respiratory infections) are hospitalised because of cough or respiratory issues occurring within the past 10 days of the onset of their illness14. ILI cases are fever and either cough or sore throat, with symptom onset within seven days. ARDS is defined as acute respiratory failure with acute onset, bilateral chest infiltrates on the radiograph, a PaO2/FiO2 below 200 (ALI < 300 vs. ARDS <200), and absence of congestive heart failure as evidenced by a wedge pressure below 18 mmHg15. KDIGO (Kidney disease: Improving Global Outcomes) defines AKI (Acute Kidney Injury) as the presence of one or more of the following: serum creatinine levels rise by 0.3 mg/dl or greater (26.5 μmol/l) within 48 h, serum creatinine levels increased by 1.5 times or more compared to the previous seven days, and urine volume is less than 0.5 ml/kg/h for at least 6 h16.
Sample collection and biomarker analysisSamples were collected from COVID-19 positive cases according to the WHO guidelines, labelled with relevant patient information and stored at -20°C. Patient’s serum samples were collected and transported in a cold chain to the King Institute of Preventive Medicine and Research (KIPMR). The samples were processed as per the laboratory standard operation protocol, and were tested at the KIPMR using the Luminex platform to quantitate the biomarkers in pmoles/l. The samples were analysed for the following biomarkers such as endothelial markers, namely VEGF, VCAM, ICAM, Galectin-3, Angiopoietin-2, Thrombomodulin, Soluble Tumour necrosis factor receptor 1 (s-TNFR1) and IL6. These biomarkers were analysed using a Human Luminex multiplex bead-based assay system (Bioplex 200, Bio-Rad, CA, USA) as per the manufacturer’s instructions.
Briefly, the samples were diluted to a dilution of 1:2 with the kit’s calibrator diluent to evaluate all markers except Galectin-3. For Galectin-3 the dilution was 1 in 50. Further, 50 µl samples/standards were added to the assay plate containing magnetic beads and incubated for 1 h at RT. After incubation, the assay plate was washed three times with wash buffer and incubated with a 50 µl detection antibody for 30 min at RT. The assay plate was rewashed and incubated with 50 µl of streptavidin-PE for 10 min at RT. After washing, the samples were resuspended in 125 µl of assay buffer. The assay plate was analysed using a plate analyser. The concentrations of the analytes were determined from standard curves tested and expressed in picograms (R&D systems, Bibiotechne, USA).
Statistical analysisThe statistical analysis was performed using GraphPad Prism 8.2.0 software (GraphPad software, San Diego, CA). Column statistics were performed, and all the quantitative variables were represented as mean ± standard deviation (SD). A one-way ANOVA was performed to compare more than two groups. Multiple comparisons were done using the Kruskal-Wallis test. The concentration of all biomarkers is expressed as pg/ml; *P<0.05 was considered significant.
ResultsAll the COVID-19-positive patients had fever, rhinorrhoea, and myalgia on presentation. The typical symptoms like fever, cough, rhinorrhoea, and body ache were present in both groups, among those who were hospitalised and those who had ILI/ home isolation. Among the infected individuals, 14 were geriatric patients (7 SARI Cases and 7 ILI Cases). Paediatric patients were not included in the study. Out of the 40 COVID-19 positive patients, 17 (42.5%) were male and 23 (57.5%) were female (Table I). Among the females, eight were antenatal cases, with one patient having a history of repeated abortions. Diabetes mellitus (65%) was the major comorbid condition associated with COVID-19 among SARI patients, followed by hypertension, anaemia, and chronic kidney disease (CKD) (Table II).VEGF levels were not significantly raised in the SARI group when compared with that of healthy controls (P=0.8804). The levels were statistically significantly different when comparing the ILI group with healthy controls (P=0.0014; Fig.1A).
Table I. Age wise distribution among patients under study
Range (yr) COVID19+ SARI (admitted patients) (n=20), n (%) COVID19+ ILI (under home isolation) (n=20), n (%) 18-29 6 (30) 7 (35) 30-54 7 (35) 6 (30) >55 7 (35) 7 (35)Table II. Comorbid conditions among study patients
Comorbid condition COVID19+SARI (n=20), n (%) COVID19+ILI (n=20), n (%) Hypertension 8 (40) 3 (15) Diabetes mellitus 13 (65) 4 (20) CKD 5 (25) - Anaemia 10 (50) 1 (5) Rheumatoid arthritis 1 (5) -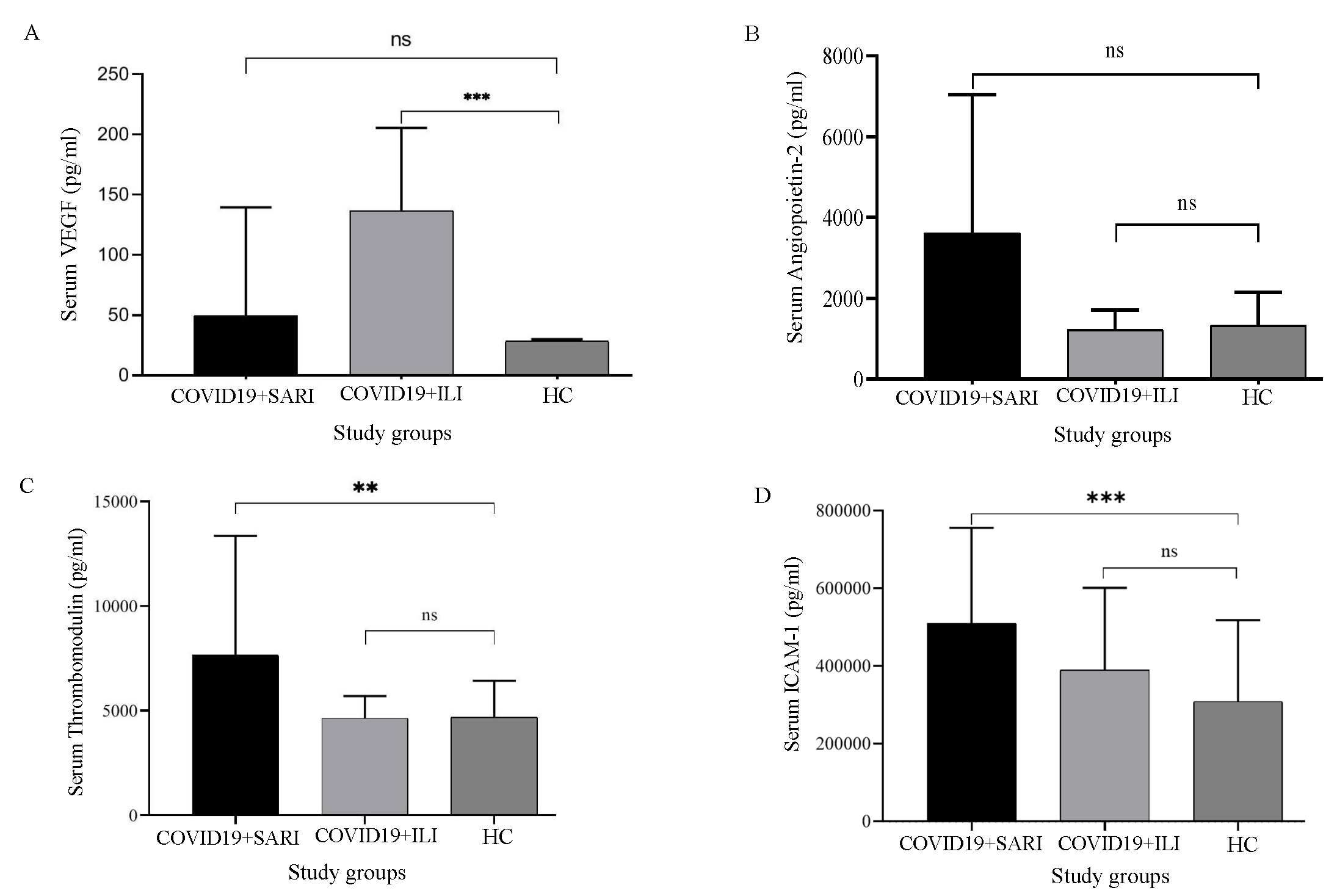
Export to PPT
The levels of angiopoietin were raised significantly higher among the SARI group when compared to that of healthy controls (P=0.0016). There was a significant elevation in the SARI group when compared to ILI cases (P=0.0015; Fig. 1B). However, angiopoietin levels in the ILI group were not significantly raised compared to those of healthy controls (P=0.5484). Thrombomodulin levels were significantly higher in SARI patients when compared to healthy controls (P=0.0024). Thrombomodulin levels were significantly higher in SARI patients when compared to ILI patients (P=0.0154). Thrombomodulin levels were not significantly raised when the comparison between ILI patients and healthy controls (P=0.2773) was done (Fig. 1C).
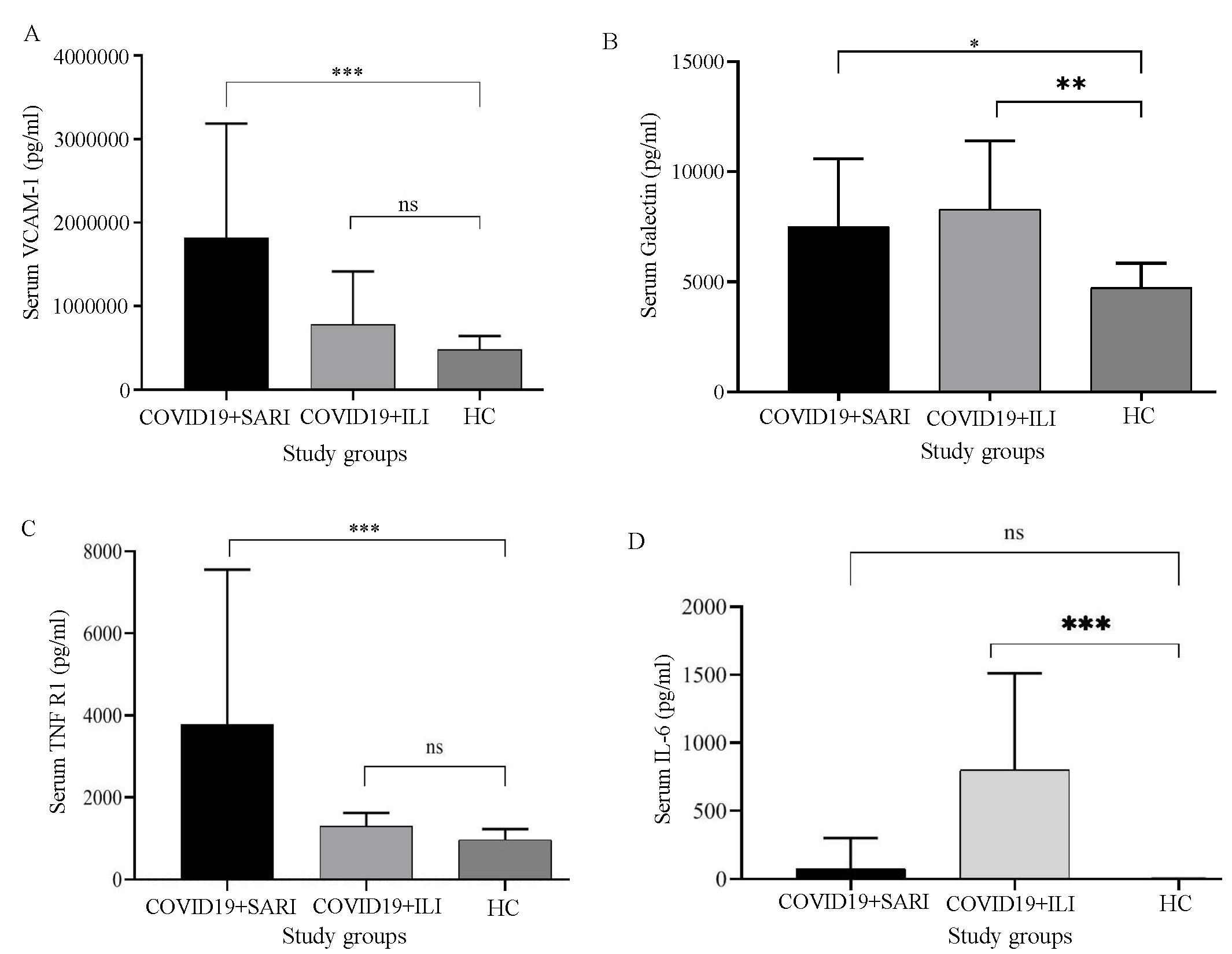
ICAM levels were elevated among ILI patients when compared to those in healthy controls (P=0.0145), which was statistically significant. It was also elevated among SARI patients when compared to that in healthy controls (P=0.0001). The ICAM was not significantly elevated between ILI and SARI patients (P=0.0775; Fig. 1D). VCAM levels were not significantly elevated among ILI patients when compared to those in healthy controls (P=0.3265). It was elevated among SARI patients when compared to that in healthy controls with P=0.0004; Fig. 2A).
Export to PPT
Among COVID-19-positive patients requiring admission, Galectin 3 levels were significantly raised compared to those in healthy controls (P=0.0063). Among COVID-19-positive ILI patients, Galectin 3 was raised considerably compared to that in healthy controls. (P=0.0360; Fig. 2B).
TNFR1 levels of ILI patients (ILI) were not significantly raised compared to that of healthy controls (P=0.9022). The levels of TNFR1 among SARI/patients requiring admission were significantly raised compared to those in healthy controls, especially among patients with several comorbidities who presented the causality (P=0.0103; Fig. 2C).
IL6 was significantly elevated in the ILI group when compared to that in healthy control (P=0.0003). It was also elevated among the SARI group when compared with healthy control; which was not statistically significant (P=>0.999; Fig. 2D).
DiscussionThe evolution and progression of COVID-19 depends on a plethora of host and agent-related factors from the strain of the virus to virus-receptor interaction. Till now, there is no specific biomarker/panel of markers that can prognosticate course of COVID-19 infection. The routinely tested markers namely CRP, and S ferritin reflect generalised inflammation, and it is up to the clinician to decipher the next step. Our findings indicate that elevated levels of VEGF, ICAM, VCAM, Angiopoietin 2, Galectin-3, Thrombomodulin, TNFR1, and IL-6 may possibly provide critical information to the treating physician about the severity and progression of COVID-19 infection17. The elevation of VEGF levels in the SARI patient group was insignificant when compared with healthy controls, whereas ILI group showed a significant rise. A recent study reported that the high circulating level of angiogenesis markers like VEGF in COVID-19 patients in a plasma profiling study18. The elevated level of VEGF-A contributes to the promotion of endothelial leakage and inflammatory cell intrusion19. ICAM levels were elevated among COVID-19-positive patients in both the ILI and SARI groups, indicating that even in mild infection, individuals experiences some degree of endothelial activation. This finding suggests that the introduction of early interventions to counter endothelial dysfunction may be beneficial in preventing disease progression, especially the introduction of antiviral/anti-inflammatory agents20. The results of a recent study demonstrated that markers like IL-6, TNF-α, ICAM-1 were associated with endothelial inflammation, and injury was present in the lungs of COVID-19 patients as compared to the H1N1 subtype 2009 cases and control patients21. VCAM levels were similar among healthy controls and ILI patients but elevated among SARI patients, potentially due to pro-inflammatory cytokines contributing to endothelial dysfunction. The soluble ICAM-1 and VCAM levels were elevated in patients with COVID-19 and changed during the progression and regression of the disease, suggesting that these inflammatory markers may serve as an effective indicator of endothelial dysfunction and inflammation in COVID-1922. This finding suggests that interventions targeting pro-inflammatory cytokines may be effective in preventing complications in SARI patients23. Angiopoietin 2 levels were significantly raised in SARI patients, reflecting inflammatory cytokine-mediated activation in the acute stage24. Hence Angiopoietin 2 levels may be a valuable biomarker to identify COVID-19 patients at risk for developing acute kidney disease and as a potential therapeutic target. Galectin-3 levels were elevated among all COVID-19 patients, indicating its potential as a diagnostic or prognostic biomarker for inflammatory and fibrotic diseases. Several reports have extensively examined Galectin-3, which has been found to impact immune system cells and contribute to inflammation, fibrosis, apoptosis, and host defense25. Galectin-3 showed significant correlations with various inflammatory and thrombo-inflammatory biomarkers, suggesting its involvement in the pathophysiology of the inflammatory response in COVID-1926. Future studies are required to explore the utility of Galectin-3 as a prognostic biomarker for COVID-19-related complications27. It was observed that thrombomodulin was significantly elevated in the SARI group, indicating an inflammatory pro-coagulant state. This finding suggests that interventions targeting the coagulation system may be effective in preventing complications in SARI patients28. TNFR1 was significantly elevated in the SARI group, suggesting its involvement in hyper inflammatory states like ARDS. A progressive SARS-CoV-2 infection is associated with several risk factors, and an uncontrolled overproduction of inflammatory cytokines contributes significantly to its pathogenesis. Some research groups assert that the baseline level of TNF is not different between mildly, severely, and critically ill COVID-19 patients, whereas others suggest that the TNF level is higher in severely ill COVID-19 patients29-32. Recent clinical studies have found that high levels of sTNFR1 are associated with chronic kidney disease progression in various clinical situations33. Furthermore, the bronchoalveolar lavage analysis showed that sTNFR1 causes cellular apoptosis in the pulmonary microvasculature during ischemic AKI34. Additionally, it was demonstrated that sTNFR1 serves as a significant predictor of overall mortality in individuals with chronic kidney disease and type 2 diabetes35. This can lead us to the understanding that TNFR1 may be a valuable biomarker for COVID-19 patients at risk for ARDS and as a potential therapeutic target as well. Finally, IL-6 levels were elevated in both study groups, indicating acute inflammation. The IL-6 plays a crucial role in the host response to local tissue infection. However, it also contributes to systemic inflammation during infection, particularly in individuals with pre-existing health conditions. This variability in the immune response may lead to differences in the clinical manifestations of COVID-19, which can be categorised based on the prevalence of lung-dominant hyperinflammation linked to pneumonia, secondary hemophagocytic lympho-histiocytosis, cardiac, and renal complications, as well as post-viral effects36.The extensive inflammatory reaction bears similarities to cytokine release syndrome, which could be the underlying cause of numerous serious complications. Notably, patients with severe COVID-19 exhibit markedly elevated serum IL-6 levels37. This finding suggests that IL-6 may be a valuable biomarker for identifying COVID-19 patients at risk for developing severe disease, as well as a potential therapeutic target. The limitations of the present study include lesser number of individuals with ILI and SARI requiring hospital admission and the low number of COVID-19 cases with serious complications. This study was done when the number of COVID-19 cases was fewer. The markers discussed will be raised in other inflammatory conditions but their role as prognostic indicators in COVID-19 is noteworthy (Table III). Identification of the increased level of biomarkers in this pilot study can pave the way for taking up studies extending on to other emerging and remerging infections challenging humans.
Table III. Analysis of biomarkers among COVID-19 with ILI/SARI/healthy controls
Parameters Remarks P values* VEGF HC vs ILI 0.001 HC vs SARI 0.880 Angiopoietin-2 HC vs ILI 0.548 HC vs SARI 0.001 ILI vs SARI 0.001 Thrombomodulin HC vs ILI 0.277 HC vs SARI 0.002 ILI vs SARI 0.015 ICAM-1 HC vs ILI 0.0145 HC vs SARI 0.0001 ILI vs SARI 0.0775 VCAM-1 HC vs ILI 0.3265 HC vs SARI 0.0004 ILI vs SARI 0.0069 Galectin-3 HC vs ILI 0.006 HC vs SARI 0.036 ILI vs SARI 0.369 TNFR1 HC vs ILI 0.9022 HC vs SARI 0.0103 ILI vs SARI 0.0031 IL-6 HC vs ILI 0.0003 HC vs SARI 0.999Overall, effective risk stratification is essential in the management of COVID-19, and our findings emphasise this necessity. We have observed that biomarkers such as ICAM and Galectin-3 are reliably elevated in all COVID-19-positive patients, making them critical for early detection and intervention. Moreover, markers like VCAM, Angiopoietin-2, Thrombomodulin, and TNFR-1 reveal heightened levels specifically in patients who require hospitalisation, underscoring their vital role in prognosis and treatment decisions. These compelling insights highlight the urgent need for further research to validate these biomarkers and fully harness their potential in combating the fatality of emerging and re-emerging infections.
Comments (0)